FIGURE 26.1 Drawing of the superior surface of an unstained horizontal section of the adult human brain, through the internal capsule, basal ganglia (BG), and thalamus.
The internal capsule thrusts itself squarely through the region of the BG, vastly complicating its anatomy and connections. Important components of the BG and extrapyramidal system lie separated on opposite sides of the capsule. The putamen and GP are lateral to it; the caudate, thalamus, STN, and SN lie medial. All efferent and many afferent connections of the BG are made with the diencephalon and midbrain, which are separated from the BG by the internal capsule and crus cerebri. The caudate and putamen are cytologically and functionally nearly identical, but the anterior limb of the internal capsule separates one from the other. Fibers of the internal capsule and crus cerebri also separate the SN from the GP, which are functionally closely related.
The caudate nucleus and putamen are semi-continuous, separated by the streaming fibers of the anterior limb of the internal capsule (Figure 26.1). The alternating gray and white strands led to the name striatum (striped). Bundles of finely myelinated, small-diameter fibers (“Wilson’s pencils”) crossing the striatum toward the GP also contribute to the marbled appearance. Inferiorly, just above the anterior perforated substance, the head of the caudate fuses with the inferior part of the putamen and is continuous medially with the nucleus accumbens. Histologically, the caudate and putamen are identical; they have a common embryologic origin. They contain a few large and many small neurons, with the small cells predominating by 20:1. Dendrites may be spiny or aspiny. The most common cell type in the striatum is small and spiny and contains gamma amino butyric acid (GABA), plus enkephalin (ENK), dynorphin, or substance P (SP). The small, spiny neurons are the primary source of striatal efferents. The small, aspiny neurons are cholinergic.
The microstructure of the striatum consists of a matrix of cells that stain histochemically for acetylcholine (ACh), with patches or islands of cells that contain other neurotransmitters. The islands are referred to as striosomes, and the striatum is a mosaic arrangement of islands or patches of striosomes lying in the matrix of cholinergic cells. The striosomes primarily contain SP and ENK. The ENKergic neurons have D2 dopamine receptors; SP neurons have D1 receptors. In the caudate, striosomes have a higher concentration of dopamine compared to the matrix. The striosome-matrix pattern is not as evident in the putamen, which consists mostly of matrix, or in the ventral striatum, which consists mostly of striosomes. The cholinergic neurons of the matrix are facilitatory to the projection neurons and are inhibited by dopamine.
The GP is medial to the putamen, just lateral to the third ventricle. An external medullary lamina separates the GP from the putamen. Internally, the GP is divided by an internal medullary lamina into a lateral, or external, zone (the globus pallidus externa, GPe); and a medial, or internal, zone (the globus pallidus interna, GPi). The GP contains only about 5% as many cells as the striatum, and all are large neurons. Neurons throughout the GP use primarily GABA as a neurotransmitter, less often ACh. The associated neuropeptide is SP in the GPi and ENK in the GPe.
The SN is a gray mass that lies in the cerebral peduncle between the crus cerebri and the tegmentum of the midbrain at the level of the superior colliculi. The SN is composed of two parts: the deep pars or zona compacta (SNc), which contains the large, melanin-containing, dopaminergic neurons that give the structure its name; and the more superficial zona reticulata (SNr), which contains large, multipolar, nonpigmented, GABAergic neurons similar to those in the GP. The SNr is closely related functionally to—essentially a midbrain extension of—the GPi; they are separated only by fibers of the internal capsule and crus cerebri, and both are involved in BG efferent functions. The SNr receives afferents through the striatonigral comb system or comb bundle, which crosses through the crus cerebri obliquely. The STN (corpus Luysii) is a small, lens-shaped gray mass situated in the ventral thalamic region just dorsal and medial to the cerebral peduncle (Figure 26.2).
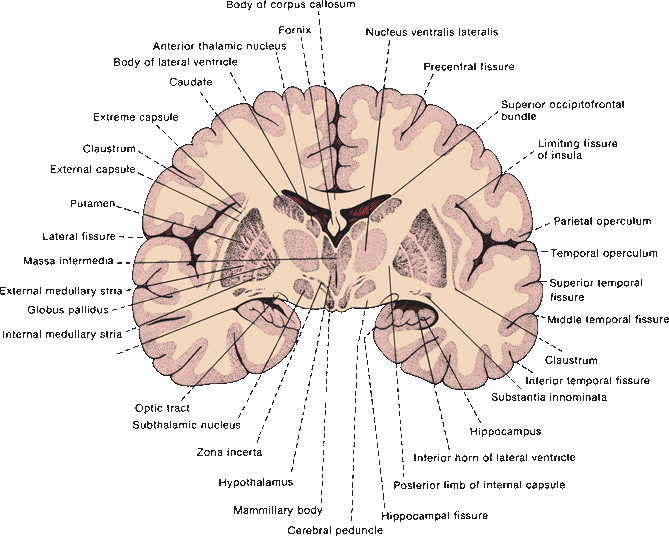
FIGURE 26.2 Drawing of the posterior surface of an unstained coronal section of the adult human brain, through the posterior limb of the internal capsule, BG, and mammillary bodies.
Other important structures involved in the extrapyramidal motor control system include the thalamus, red nucleus (RN), the brainstem reticular formation (RF), the inferior olivary nucleus in the medulla, the zona incerta (ZI), the vestibular nuclei, the pedunculopontine nucleus (PPN), and the gray matter of the quadrigeminal plate. Several thalamic nuclei are involved, and these are sometimes referred to as the motor thalamus, including the nucleus ventralis lateralis (VL), pars oralis (VLo); ventralis lateralis, pars caudalis (VLc); ventralis posterior lateralis, pars oralis (VPLo); and portions of ventralis anterior (VA). The RN is located in the tegmentum of the midbrain at the level of the superior colliculi. It has magnocellular (large-celled) and parvocellular (small-celled) portions. The caudal magnocellular part gives rise to the rubrospinal tract, and the parvocellular part to the central tegmental tract. The lateral and medial nuclear groups of the RF are situated in the tegmentum of the midbrain, and other constituents of the RF that either inhibit or facilitate motor responses are placed caudally in the brainstem. The PPN is a cholinergic nucleus that lies caudal to the SN in the brainstem tegmentum, partially buried in the superior cerebellar peduncle. It receives afferents primarily from GPi/SNr and sends cholinergic projections to the dopaminergic neurons in the SNc. It may be involved in locomotion. Patients with Parkinson’s disease have significant loss of PPN neurons, and dysfunction of the PPN may be important in the pathophysiology of the locomotor and postural disturbances of parkinsonism.
The BG have rich connections with one another and with brainstem structures, as well as with the cerebral cortex and with lower centers (Figure 26.3). The area of the cortex involved is the motor cortex, which includes the precentral motor regions including the supplementary and premotor areas. In essence, the cerebral cortex projects to the striatum, which in turn projects to the GP and SNr; efferents go to the thalamus, which then projects back to cerebral cortex, primarily to the motor areas. Table 26.1 summarizes some of the major connections.
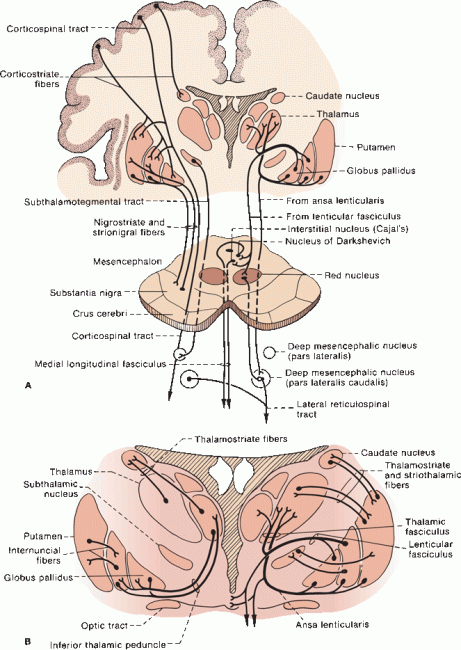
FIGURE 26.3 A. Principal connections of the BG. The thalamocortical portions of the loops (cortex-BG-thalamus-cortex) are omitted. B. Detail giving connections between the BG and thalamus.
TABLE 26.1 Major Basal Ganglia Pathways
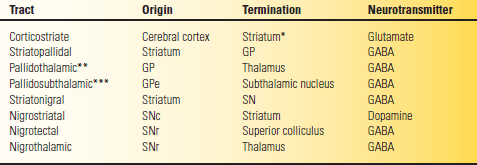
*The dorsal striatum, the caudate, and putamen.
**Via the fasciculus and ansa lenticularis.
***Via the subthalamic fasciculus.
GABA, gamma amino butyric acid; GP. globus pallidus; SN, substantia nigra; SNc, pars compacta; SNr, pars reticulata.
Striatal Afferents
The striatum receives topographically organized glutaminergic axons that originate from small pyramidal cells in layers V and VI of the entire ipsilateral neocortex. The striatum also receives afferents from the thalamus. These connections provide the striatum with sensory and cognitive inputs. The head of the caudate receives projections from the frontal lobe, the body from the parietal and occipital lobes, and the tail from the temporal lobe. The massive input from the frontal lobe to the head is the reason it is so much larger than the remainder of the nucleus. These connections form the anatomical substrate for the role of the caudate in cognition. The caudate also receives fibers from the dorsomedial (DM) and VA nuclei of the thalamus (thalamostriate fibers) and from the putamen.
The connections of the putamen are more focused; it receives fibers from cortical areas 4 and 6 and from the parietal lobe, the perirolandic motor centers, which are essentially the same areas that give rise to the corticospinal tract. It has a large connection with the caudate. It also receives fibers from the SN by the comb bundle.
Striatal Efferents
The caudate sends fibers to the thalamus (striothalamic fibers) and to the putamen and GP. The primary efferent fibers from the striatum project to the GP and SN. The striatopallidal fibers from the caudate run directly through the anterior limb of internal capsule, and those from the putamen project medially through the external medullary lamina into the GP.
Pallidal Afferents
The principal afferents to the GP are from the caudate and putamen. The STN also sends fibers to the GP in the subthalamic fasciculus; some pass through the internal capsule into the medial segment of the GP, some cross in the supraoptic commissure (of Gudden). There are also fibers from the SNc to the GP. The GP also receives impulses from DM and VA, through thalamostriate fibers in the inferior thalamic peduncle, and from area 6 and possibly area 4 through corticospinal collaterals.
Pallidal Efferents
The pallidal efferents are the principal outflow of the BG. There are four major bundles: (a) the fasciculus lenticularis; (b) the ansa lenticularis; (c) pallidotegmental fibers, which arise from GPi; and (d) pallido-subthalamic fibers, which arise from GPe. Pallidofugal fibers are often discussed in terms of their relationships to the “fields of Forel.” August Forel performed some of the early anatomical studies of the BG and subthalamic region using degeneration techniques. (Although his name is inextricably linked to the neuroanatomy of the subthalamic region, his principal reputation arises from seminal studies of the social behavior of ants.) Forel identified fields or regions in the subthalamic and BG area and labeled them “H” for “Haubenregionen” because of their resemblance to a hat plume. There are Forel fields H, H1, and H2; these refer to different fiber bundles that course in the region of the RN, STN, and ZI. The prerubral or tegmental field (Forel field H) lies just rostral to the RN, and primarily contains dentatothalamic fibers from the contralateral superior cerebellar peduncle and rubrothalamic fibers from the ipsilateral RN ascending toward the thalamus through the subthalamic region. The dentatothalamic and rubrothalamic fibers form a capsule around the RN; the portion of this capsule just rostral to the RN is the prerubral field. Pallidofugal fibers stream into the prerubral field and then turn upward. As these fibers ascend toward the thalamus, the prerubral field divides into dorsal and ventral lamina; the dorsal division consists of the lenticular fasciculus (Forel field H2), and the ventral division is the thalamic fasciculus (Forel field H1).
Both the ansa and fasciculus lenticularis have the same origin, GPi, and the same destination, the thalamus; the difference is that the fasciculus penetrates through, and the ansa curves around, the internal capsule. Both pass through field H, and both join with the thalamic fasciculus. The fasciculus lenticularis emerges from the dorsal surface of GPi, and then pierces the internal capsule to lie just above the STN and below the ZI. The ansa lenticularis emerges from the ventral surface of GPi, runs ventromedially to loop around the posterior limb of the internal capsule, and then enters the prerubral field. After traversing the internal capsule, the fasciculus lenticularis joins the ansa lenticularis at the medial border of the ZI; both then enter the thalamic fasciculus just above the ZI.
The thalamic fasciculus (Forel field H1) is a complex bundle that lies just dorsal to the ZI. It carries pallidofugal fibers as well as rubrothalamic and dentatothalamic fibers. The thalamic fasciculus enters the rostral ventral tier of thalamic nuclei, primarily VL and VA. Some pallidofugal fibers separate from the thalamic fasciculus and enter the centromedian thalamic nucleus. The dentatothalamic fibers in the thalamic fasciculus are destined primarily for VL, but some enter the intralaminar nuclei. The VL is involved in integration and coordination of BG and cerebellar function. The VL in turn projects to area 4 of the motor cortex. The point where the thalamic fasciculus converges on VL is of singular strategic importance in the function of the motor system.
Subthalamic Nucleus
The STN is reciprocally connected with the GP via the subthalamic fasciculus, a bundle that runs directly through the internal capsule. The connection to the STN is the only pallidal efferent to arise from GPe; all others come from GPi. The STN sends fibers back to GPe, as well as to GPi, through the subthalamic fasciculus.
Substantia Nigra
The SN extends from the pons to the subthalamic region and makes up the primary dopaminergic cell population of the midbrain; cholinergic cells are also present. The SNc of one side is continuous across the midline with the SNc of the opposite side. Cells of the SNc contain neuromelanin, a by-product of dopamine synthesis. The striatonigral afferents from the striatum use GABA and either SP or enkephalin as transmitters. The SNr receives strionigral fibers from the striatum, GP, and STN. The primary efferents from the SN are to the striatum, midbrain tectum, and thalamus (Table 26.1). The SNr is functionally related to the GPi; its efferents are GABAergic. Dopaminergic nigrostriatal fibers from SNc project to the striatum. The nigrothalamic tract runs to VA and DM. The nigrotectal tract connects the SN with the ipsilateral superior colliculus and may be involved in the control of eye movements. There are also connections between the SN and the PPN and RF.
BASAL GANGLIA PHYSIOLOGY
The connections of the motor system are complex (Figure 22.2). There are two major loops: the BG and the cerebellar. The essential connections in the BG loop are cortex → striatum → globus pallidus → thalamus → cortex. The projections of the thalamus, cortex, and STN are excitatory; the outputs of the striatum and pallidum are primarily inhibitory. The projections from the cortex to the striatum and from the thalamus to the cortex are both excitatory (glutaminergic). The pathway from the striatum to the thalamus may be either excitatory or inhibitory depending on the route. Current models of BG function include a direct and an indirect loop or pathway for the connection between the striatum and thalamus (Box 26.1). In brief, the direct loop is excitatory and the indirect loop is inhibitory. The indirect loop brings in GPe and STN, which are not involved in the direct pathway (Figure 26.4). The caudate, putamen, and STN make up the BG input nuclei; the GPi and SNr are the output nuclei. The input and output nuclei are connected by the direct and indirect loops. In essence, the output nuclei, GPi and SNr, tonically inhibit the motor thalamus; the input nuclei either facilitate cortical motor activity by disinhibiting the thalamus, or inhibit motor activity by increasing thalamic inhibition. The direct pathway serves to facilitate cortical excitation and carry out voluntary movement. The indirect pathway serves to inhibit cortical excitation and prevent unwanted movement. Disease of the direct pathway produces hypokinesia, for example, parkinsonism; disease of the indirect pathway produces hyperkinesias, for example, chorea or hemiballismus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







