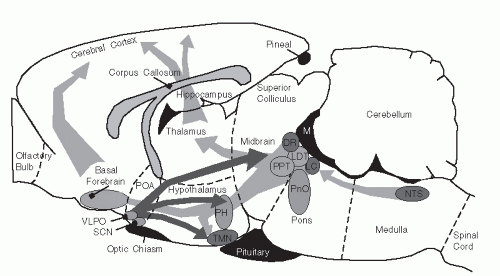
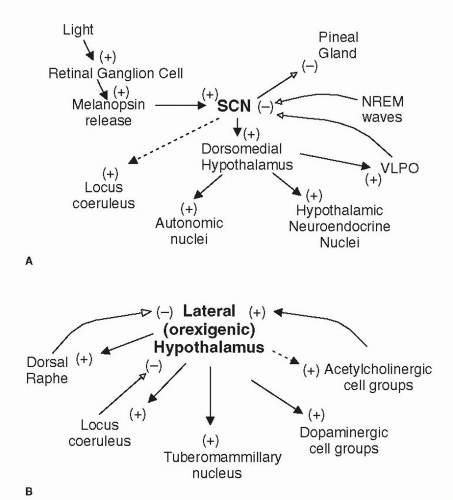
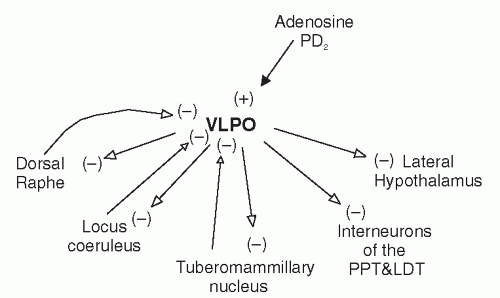
A number of diverse explanations for the primary function of sleep have been submitted. A few of the more prominent ones are reviewed here.
Energy Conservation Theories
In 1975, Berger put forward that the goal of sleep is to reduce the metabolic rate below that obtained from resting alone (
10). Attention is drawn to similarities between the slow waves of sleep and the EEG during entry into hibernation and shallow torpor. The suggestion was that these phenomena share a common purpose: to save energy. Among other detractors though, great doubt is cast on this theory by the realization that, at best, sleep has a metabolic savings of 15%, and more likely of just 5% to 10% (
11,
12).
A second metabolic theory to emerge from the study of mammalian sleep states is that the goal of sleep is to enforce periods of inactivity and thus keep energy expenses at an affordable rate relative to energy intake. In summary, sleep quotas for different species were correlated with body mass, brain weight, food (including caloric) intake, and the type of diet. Multiple regression analyses showed that brain weight and body weight could account for up to about 30% of the variance in sleep time among various mammalian species (
1,
13,
14). However, based on this theory alone, the need for sleep is still far from totally accounted for.
Memory Reinforcement
A currently popular belief is that sleep evolved as an adaptation to the increasing demands placed on the brain as more time became required to process complex sensory information, especially vision. Time was also needed to maintain the now vast stores of sensory and motor memories (
8). By adopting sleep, neural circuits used during wakefulness to acquire new input could be temporarily turned over to processing and storing (memorizing) the information during sleep.
The slow waves of NREM sleep are thought to reinforce individual neural components of complex memory circuits and also the links between the components. Because individual components rather than the entire complex memory are reinforced, it is referred to as “uncoordinated reinforcement.” This is done without fast waves and so without awareness and, therefore, avoids having to simultaneously process new sensory input. NREM sleep is thought to be the earliest form of sleep, perhaps evidenced by its presence in reptiles. It would have been associated with behaviors such as closing the eyelids and retiring to secure, dimly lit resting sites.
During further evolution toward warm-bloodedness, mechanisms continued to develop to encourage uninterrupted sleep during stronger stimuli, such as higher arousal thresholds and further reduced muscle tone to the point of atonia, especially in antigravity muscles. At this point, it was possible to allow fast waves (often associated with wakefulness) to participate in sleep. Fast waves, the principal component of REM sleep, are thought to bind the components of a particular memory together, that is, to form a so-called coordinated reinforcement of memory. These waves are used during REM sleep to reinforce both sensory (particularly visual) and motor circuits. Now, motor systems could be activated during sleep without waking the animal with muscle contractions because muscle contractions were inhibited. Reinforcement of the motor circuits during sleep was thought to be important for the survival of warm-blooded animals (
8). With their thermoregulatory capabilities, they were able to move over a greater diversity of terrains 24 hours a day.
It is proposed, therefore, that REM sleep developed along with warm-bloodedness. In agreement, it is found only in warm-blooded animals. However, a number of avian and mammalian species do not exhibit REM—mostly those exhibiting unihemispherical sleep (
8). It is suggested that because unihemispherical sleep is often accompanied by continual movement, such as swimming in the aquatic species and perhaps flying in birds, there is no need for REM sleep to further reinforce muscle movements.
In support of the memory consolidation theory is evidence that rats presented with novel stimuli show relevant spatiotemporal activity in the stimulated neuronal ensemble for up to 48 hours after the stimulus was presented (
15). Reverberation of activity was strongest during slow-wave sleep, less during waking, and variable during REM sleep. It is suggested by this group that sustained neuronal reverberation during slow-wave sleep and generelated neuronal plasticity during REM contribute to the memory-consolidating role of sleep.