, Jean Paul G. Vonsattel2, Helmut Heinsen3, 4 and Horst-Werner Korf5
(1)
Dr. Senckenbergisches Chronomedizinisches Institut, Goethe University Frankfurt, Frankfurt, Germany
(2)
Medical Center Neurological Institute, Columbia University, New York, NY, USA
(3)
Division Psychiatic Clinic Morphological Brain Research Unit, Julius Maximilians University Würzburg, Würzburg, Germany
(4)
University of Sao Paulo Medical School, Sao Paulo, Brazil
(5)
Dr. Senckenbergisches Chronomedizinisches Institut, Goethe University, Frankfurt, Frankfurt, Germany
2.1 Neuropathological Base for the Grading System of Huntington’s Disease (HD)
The deleterious action of the unstable CAG repeat expansion in the Huntington’s disease (HD) gene (also called IT15) located on the short arm of chromosome 4 involves widespread areas of the brain including sites of increased vulnerability or sites that are relatively resistant, but not spared (Andrew et al. 1993; Duyao et al. 1993; Myers et al. 1991; The Huntington’s disease Collaborative Research Group 1993). Therefore, the intensity of the degenerative process of HD is topographically variable. The neuropathological phenotype of adult HD can be almost cryptic or outstanding within the same nucleus, but at different sites. Indeed, the expression of the degenerative process differs not only among distinct anatomical compartments, but also within specific brain compartments (e.g., cerebral cortex, white matter, striatum, pallidum, thalamus, brainstem, cerebellum), or systems (e.g., basal ganglia, limbic system) (Fig. 1.4) (Braak and Braak 1992a, b; Bruyn et al. 1979; De la Monte et al. 1988; Dom et al. 1976; Dunlap, 1927; Duyao et al. 1993; Estrada-Sanchez and Rebec 2013; Fennema-Notestine et al. 2004; Ferrante et al. 1987; Hedreen et al. 1991; Heinsen et al. 1992, 1994, 1996, 1999; Heinsen and Rüb 1997; Lange 1981; Lange and Aulich 1986; Lange et al. 1976; Myers et al. 1988; Rüb et al. 2013a, 2014a, b; Selemon et al. 2004; Shoulson and Young 2011; Sotrel et al. 1991; Vogt and Vogt 1920, 1942; Vonsattel 2008; Vonsattel and DiFiglia 1998; Vonsattel et al. 1985). Furthermore, the expression of the pathological phenotypes depends on a constellation of influences driven mainly by epigenetic factors, genetic modifiers, duration of symptoms, or the idiosyncratic longevity of the patients (Hodges et al. 2006; U.S.-Venezuela Collaborative Research Project and Wexler 2004). Thus, upon postmortem examination the pathological phenotypes of HD brains are more or less obvious depending on the sites or systems that are considered and on the techniques applied for assessing the brains. The involvement and the evolution of the neurodegenerative changes in the striatum (caudate nucleus, putamen), and pallidum (paleostriatum) strikingly underscore the differential regional vulnerability occurring in HD within this discrete, relatively small subregions of the brain (Fig. 1.4) (Birnbaum 1941; Braak and Braak 1992a, b; Bruyn et al. 1979; De la Monte et al. 1988; Dom et al. 1976; Dunlap 1927; Estrada-Sanchez and Rebec 2013; Fennema-Notestine et al. 2004; Ferrante et al. 1987; Forno and Jose 1973; Hedreen et al. 1991; Heinsen et al. 1992, 1994, 1996, 1999; Heinsen and Rüb 1997; Hodges et al. 2006; Kiesselbach 1914; Landwehrmeyer et al. 1995; Lange 1981; Lange and Aulich 1986; Lange et al. 1976; Lewy 1923; McCaughey 1961; Myers et al. 1988; Neustaedter 1933; Roos et al. 1985; Rüb et al. 2013a, b, 2014a, b; Schroeder 1931; Selemon et al. 2004; Sotrel et al. 1991; Terplan 1924; Vonsattel 2008; Vonsattel and DiFiglia 1998; Vonsattel et al. 1985).
The vulnerability of the striatum correlates with the size of the HD-IT15 CAG repeat expansion (Furtado et al. 1996; Penney et al. 1997). The primary HD-related atrophy might be exacerbated in some instances by the age-related involution of the brain notably in patients with the lower end of the HD-IT15 CAG repeat expansion or with a concomitant Alzheimer’s disease (AD)-related brain pathology. The mechanisms causing this increased vulnerability of the striatum for the pathological process of HD are unknown.
Because the striatum of HD patients shows regional variation of the severity and progression of the degenerative process, a grading of the HD–associated striatal neuropathology was developed. This grading is based on the fact that the gradual atrophy of the neostriatum which is due to the slowly ongoing loss of neurons and the concomitant occurrence of reactive astrogliosis with differential regional expression over time has been for a long time regarded as the major neuropathological hallmark of HD.
The aim of grading the neuropathological severity is to sort and to pool samples of the striata of HD patients for research, which share comparable “Gestalt” patterns of the parenchyma. Indeed, the grading identifies striatal samples with subregions that are relatively spared (e.g., accumbens nucleus), or subregions in which the neuronal loss and reactive astrocytosis are moderate, or subregions in which the residual cells mainly consist of reactive astrocytes, oligodendrocytes, and microglial cells. Investigations focusing on the reasons for the vulnerability of the HD striatum increasingly require semiquantitative measures of the degenerative process, which are also useful for studies correlating striatal changes with changes outside of the striatum in the brains of HD patients or for clinicopathological correlations. The reproducible, rather simple grading system is widely used for studies focusing on the regional and cellular susceptibility to the toxic effects of the HD-IT15 CAG repeat expansion, which are operating directly within or indirectly outside the striatum. To some extent this grading system allows for a selection of brain samples for research with a reasonable estimation of the type of residual cells they contain (e.g., mixed glial and neuronal populations or an overwhelming glial population) (Vonsattel 2008; Vonsattel and DiFiglia 1998; Vonsattel et al. 1985).
The neuropathological HD grading provides these semiquantitative measures of the striatum at both the macroscopical (gross) and microscopical levels. Grossly the striatum is assessed by the use of three standardized thick frontal slices through the cerebral hemispheres (Figs. 2.1, 2.2, 2.3, 2.5, and 2.6), while microscopical evaluation is performed on tissue sections through four different levels of the striatum of HD patients (Figs. 2.2, 2.3, 2.4 2.5, 2.6, 2.7, and 2.8).
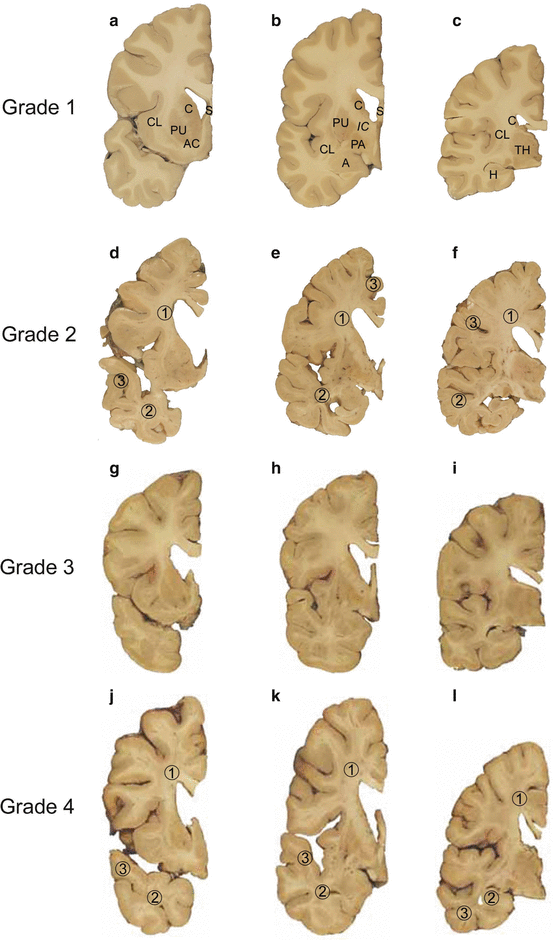
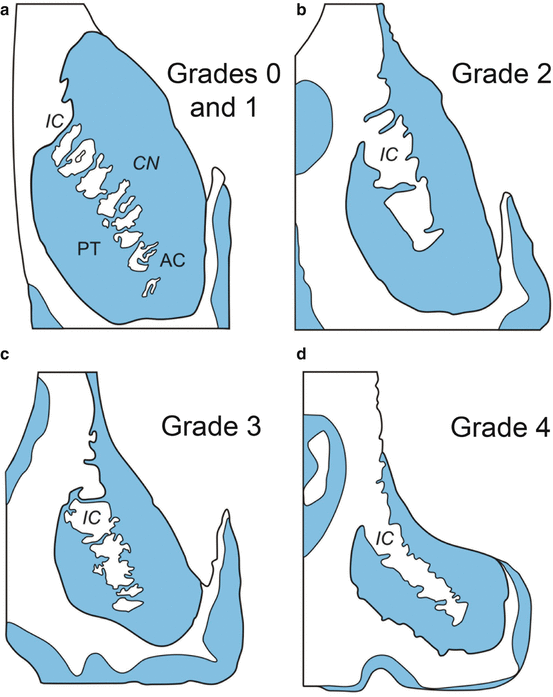
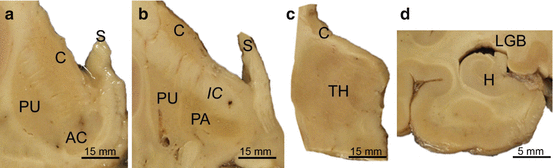
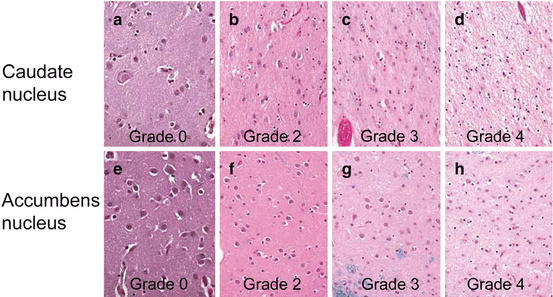
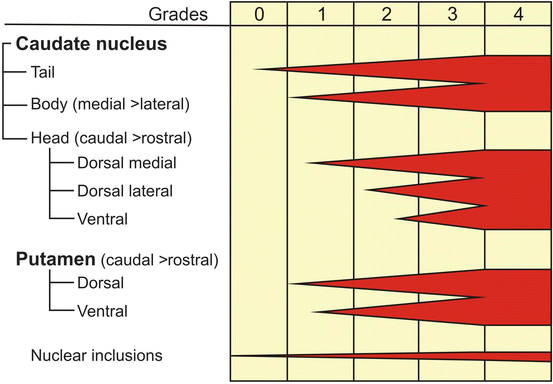
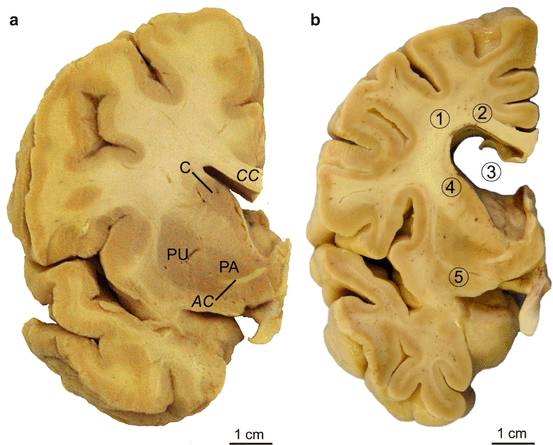
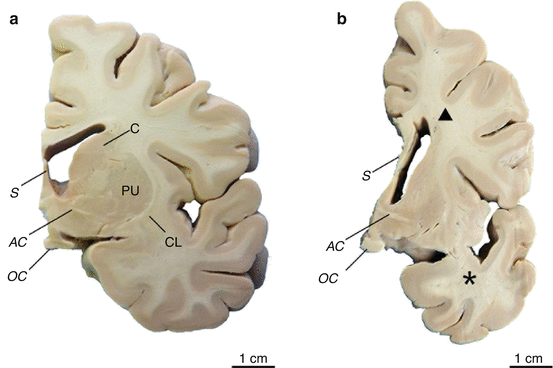
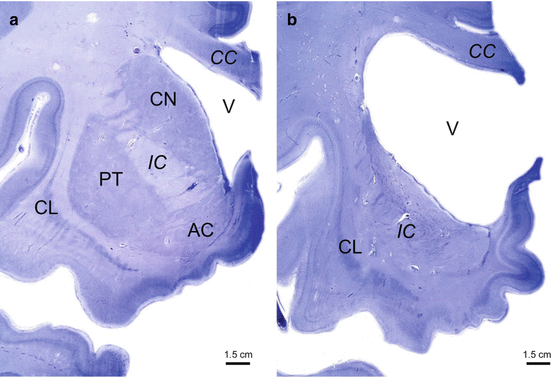

Fig. 2.1
Neuropathological grading of Huntington’s disease (HD). (a–c) Grade 1 of neostriatal atrophy in HD. Thick frontal sections through the brain of a 29-year-old male Huntington’s disease (HD) patient. The male HD patient received the mutated HD gene from his mother, suffered from motor clumsiness and gait changes, showed aggressive behavior, and died from the results of a fatal accident. (a) Rostral caudate nucleus (C), claustrum (CL), putamen (PU), and accumbens nucleus (AC) (level CAP). (b) Pallidum (PA) with the caudal portion of the head of the caudate nucleus and the adjacent putamen (level GP). (c) Lateral geniculate body of the thalamus (TH) with the body (dorsal) and tail of the caudate nucleus (ventral) (level caudal thalamus). Macroscopical changes of the brain of the patient corresponding to grade 1 of neostriatal atrophy: Atrophy of the body of the caudate nucleus and reduction of its tail (b, c). Normal gross aspect of the putamen and accumbens nucleus (a–c). (d–f) Grade 2 of neostriatal atrophy in HD. Thick frontal sections through the brain of an 89-year-old male HD patient with 17 CAG trinucleotide repeats in the normal HD gene and 40 CAG repeats in the mutated HD gene. This HD patient received the mutated HD gene from his mother HD. HD onset in this patient was at age 75, and definite involuntary choreatic movements first appeared at age 79. (d) Rostral caudate nucleus, putamen, and accumbens nucleus (level CAP). (e) Pallidum with the caudal portion of the head of the caudate nucleus and the adjacent putamen (level GP). (f) Lateral geniculate body of the thalamus with the body (dorsal) and tail of the caudate nucleus (ventral) (level caudal thalamus). Macroscopical brain changes corresponding to grade 2 of neostriatal atrophy: evident gross striatal atrophy and enlarged lateral ventricles (d–f). Despite marked atrophy of the head of the caudate nucleus, the convex outline of its ventricular surface is still retained (d). The involvement of the body and tail of the caudate nucleus is still discrete (e, f). The putamen and pallidum show an obvious volume loss (d, e), while the accumbens nucleus appears macroscopically normal (d). Some of the non-striatal atrophic changes of the brain of the patient are in part due to a concomitant Alzheimer’s disease (AD)-related pathology. Note the additional (1) cerebral white matter loss, (2) atrophy of the temporal lobe, and (3) reduction of the gray band of the cerebral cortex (d–f). (g–i) Grade 3 of neostriatal atrophy in HD. Thick frontal sections through the brain of a 40-year-old female HD patient with 17 CAG trinucleotide repeats in the normal HD gene and 49 CAG repeats in the mutated HD gene. HD onset was at age 32. The patient received the gene via paternal transmission. (g) Rostral caudate nucleus, putamen, and accumbens nucleus (level CAP). (h) Pallidum with the caudal portion of the head of the caudate nucleus and the adjacent putamen (level GP). (i) Lateral geniculate body of the thalamus with the body (dorsal) and tail of the caudate nucleus (ventral) (level caudal thalamus). Macroscopical brain changes corresponding to grade 3 of neostriatal atrophy: severe atrophy of the caudate nucleus with shrunken head, barely distinguishable body (dorsal) and tail (ventral) (g–i). The medial outline of the caudate nucleus forms a straight-line configuration, which parallels the anterior limb of internal capsule (g–i). The accumbens nucleus still has a normal appearance (g). (j–l) Grade 4 of neostriatal atrophy in HD. Thick frontal sections through the brain of a 47-year-old female HD patient with 17 CAG trinucleotide repeats in the normal HD gene and 54 CAG repeats in the mutated HD gene. HD onset was at age 29. (j) Rostral caudate nucleus, putamen, and accumbens nucleus (level CAP). (k) Pallidum with the caudal portion of the head of the caudate nucleus and the adjacent putamen (level GP). (l) Lateral geniculate body of the thalamus with the body (dorsal) and tail of the caudate nucleus (ventral) (level caudal thalamus). Macroscopical brain changes corresponding to grade 4 of neostriatal atrophy: severely atrophic neostriatal caudate nucleus and putamen with markedly concave medial outlines of the head of the caudate nucleus and adjacent internal capsule (j–l). In HD grade 4 individuals, neostriatal neuronal loss commonly amounts to 95 % and leaves behind a vacuolated neuropil in most affected regions of the neostriatum. The pallidum likewise is severely degenerated and sustains neuronal loss. Although remaining relatively preserved in at least 50 % in individuals with striatal grade 4 neuropathology, the accumbens nucleus in these individuals is by no means spared. Note the additional severe (1) degeneration of the cerebral white matter, (2) atrophy of the temporal lobe, and (3) reduction of the gray band of the cerebral cortex (j–l). Abbreviations: A amygdala, AC accumbens nucleus, C caudate nucleus, CL claustrum, H hippocampus, IC internal capsule, PA pallidum, PU putamen, S septum, TH thalamus

Fig. 2.2
Progressive degeneration of the striatum in Huntington’s disease (HD). (a–d) Schematized frontal sections through the rostral level of the striatum with the head of the caudate nucleus (CN), putamen (PT), and accumbens nucleus (AC) (level CAP). (a) Grades 0 and 1 of striatal atrophy in Huntington’s disease (HD): normal gross aspect of the neostriatum (CN and PT) and AC. (b) Grade 2 of striatal atrophy in HD: markedly atrophic head of the CN. The convex outline of the ventricular surface of the CN, however, is still retained. The PT already shows an obvious volume loss, while the AC appears macroscopically normal. (c) Grade 3 of striatal atrophy in HD: moderate to severe atrophy of the neostriatum. The medial outline of the CN is now flat and forms a nearly straight-line configuration, which parallels the anterior limb of the internal capsule (IC). (d) Grade 4 of striatal atrophy in HD: very severe atrophic CN and PT with markedly concave medial outline of the head of the CN and IC. The AC of grade 4 individuals is also atrophic (Modified according to Vonsattel et al. (1985), (Figure 2, page 566); with kind permission from Wolters Kluwer Health). Abbreviations: AC accumbens nucleus, CN caudate nucleus, IC internal capsule, PT putamen

Fig. 2.3
Standardized brain tissue blocks for the macroscopical evaluation of the grade of striatal atrophy in Huntington’s disease (HD). The assignment of a grade of the severity of the striatal pathology in the brains of Huntington’s disease (HD) patients is based on macroscopical and microscopical investigations of the striatum. The macroscopical investigation includes three standardized thick frontal brain slices that comprise four distinct levels of the striatum: (1) CAP level with the caudate nucleus, accumbens nucleus, and putamen (see Fig. 2.1a, d, g, j). (2) GP level with the pallidum and caudal portion of the head of caudate nucleus, as well as the adjacent putamen (see Fig. 2.1b, e, h, k). (3) Caudal level of the thalamus with the lateral geniculate body, as well as the body of the caudate nucleus (see Fig. 2.1c, f, i, l). For the microscopical examination, four blocks are obtained from the following thick frontal slices: (a) CAP level. (b) GP level. (c) Caudal level of thalamus through the centromedian-parafascicular complex and the body of the caudate nucleus. (d) Level of the hippocampal formation at the thalamic lateral geniculate body with the tail of the caudate nucleus. Figures (a–d) are taken from the 40-year-old female HD grade 3 patient of Fig. 2.1. Abbreviations: AC accumbens nucleus, C caudate nucleus, H hippocampus, IC internal capsule, LGB lateral geniculate body of the thalamus, PA pallidum, PU putamen, S septum, TH thalamus

Fig. 2.4
Microscopical changes in the striatum in Huntington’s disease (HD). (a, b, c, d) Luxol fast blue treated tissue sections counterstained with hematoxylin and eosin (LHE) through the head of the caudate nucleus (dorsal third at midpoint between the ependyma and the medial edge of the anterior limb of the internal capsule). (e, f, g, h) LHE treated tissue sections through the accumbens nucleus (ventral third, at midpoint along its horizontal axis) (magnification: 400×). (a) Regular nerve cell density in the head of the caudate nucleus and (e) accumbens nucleus of an 80-year-old male control individual. (b) Definite nerve cell loss and reactive astrogliosis in the head of the caudate nucleus and (f) unremarkable accumbens nucleus of an 89-year-old male Huntington’s disease (HD) patient with grade 2 of striatal atrophy (see also Fig. 2.1d–f). (c) Severe neuronal loss and reactive astrogliosis in the head of the caudate nucleus and (g) not more than mildly decreased neuronal density in the accumbens nucleus of a 40-year-old female HD patient with grade 3 striatal atrophy (see also Fig. 2.1g–i). (d) Nearly complete neuronal loss, vacuolated neuropil, and reactive astrogliosis in the head of the caudate nucleus and (h) decreased neuronal density in the accumbens nucleus of a 47-year-old female HD patient with grade 4 striatal atrophy (see also Fig. 2.1j–l)

Fig. 2.5
Progression of striatal neuronal loss and astrogliosis in Huntington’s disease (HD). Schematic diagram summarizing the neuropathological grades and the topographical variation and progression of neostriatal neuronal loss and astrogliosis characteristic of Huntington’s disease (HD). The gradients of neuronal loss decrease along the caudo-rostral, mediolateral, and dorsoventral axes of the neostriatum, while the gradients of the reactive astrogliosis increase along these neostriatal axes. Owing to the extent of degeneration, these neuropathological gradients are often blurred in grade 4. Neuronal nuclear inclusions of the disease protein huntingtin (HTT) may evolve already in the absence of striatal neurodegeneration (grade 0) and their formation progresses during the neuropathological HD grades 1–4 (Modified according to Vonsattel and DiFiglia (1998), (Figure 6, page 375); with kind permission from Wolters Kluwer Health)

Fig. 2.6
Atrophy of the striatum and white matter loss in Huntington’s disease (HD). (a) Frontal section through the basal forebrain of a representative control individual. (b) Frontal section through the left basal forebrain of a clinically diagnosed and genetically confirmed Huntington’s disease (HD) patient. Note the loss of deep white matter (1), the narrowed corpus callosum (CC) (2), and widened third ventricle (3). The atrophy of the caudate nucleus (C) (4) and putamen (PU) (5) corresponds to Vonsattel grade 2 of neostriatal atrophy (Reprinted from Rüb et al. (2009), (Figure 2, page 6); with kind permission from John Wiley and Sons). Abbreviations: AC anterior commissure, C caudate nucleus, CC corpus callosum, PA pallidum, PU putamen

Fig. 2.7
Degeneration of the striatum and white matter loss in Huntington’s disease (HD). (a) Frontal section through the basal forebrain of a representative control individual at the level of the optic chiasm (OC) with the caudate nucleus (C) and putamen (PU). (b) Frontal section through the same level of the left basal forebrain of a clinically diagnosed and genetically confirmed male Huntington’s disease (HD) patient. The atrophy of the C and PU of this HD patient corresponds to Vonsattel grade 2 of neostriatal atrophy. Note the additional atrophy of the temporal lobe (asterisk) and loss of deep white matter (triangle). Abbreviations: AC anterior commissure, C caudate nucleus, CL claustrum, PU putamen, OC optic chiasm, S septum

Fig. 2.8
Neuronal loss in the neostriatum in Huntington’s disease (HD). (a) Frontal section through the rostral portion of the left striatum of a representative control individual with the neostriatal caudate nucleus (CN) and putamen (PT), as well as with the accumbens nucleus (AC) of the limbic striatum. (b) Atrophy, pallor, and severe neuronal loss in the neostriatal CN and PT of a representative Huntington’s disease (HD) patient with Vonsattel grade 4 of neostriatal atrophy. Note the markedly enlarged lateral ventricle (V), the atrophic internal capsule (IC), as well as the markedly concave medial outlines of the CN and IC. Although also atrophic and pale, the AC of the limbic striatum of the HD patient was less severely affected by neuronal loss than the neostriatal CN and PT. (a, b: gallocyanin staining, 400 μm gelatine sections). Abbreviations: AC accumbens nucleus, CC corpus callosum, CL claustrum, CN caudate nucleus, IC internal capsule, PT putamen, V lateral ventricle
2.2 Grading of Striatal Neuropathology in Huntington’s Disease (HD)
The striatal neurodegeneration of HD has an ordered and topographic evolution and distribution. Briefly, along the sagittal axis of the brain, the tail of the caudate nucleus shows more degeneration than the body of the caudate nucleus, which in turn is more involved than the head of the caudate nucleus. Likewise, the caudal portion of the putamen is more degenerated than the rostral portion. Along the coronal axis of the brain, the dorsal regions of the striatum are more involved than the ventral ones and the medial ones more than the lateral ones. Thus, from a three-dimensional point of view, the worsening of the degenerative process in the striatum, which encompasses neuronal loss and reactive astrogliosis, appears to simultaneously move in a caudo-rostral direction and concomitantly, in both, the dorsoventral and mediolateral directions. Gradually a regional, differential, and slowly decreasing density of neostriatal neurons becomes inversely proportional to the density and severity of the slowly emerging reactive astrogliosis, which parallels the severity of the neuronal loss. Most residual neurons in the striatum have normal somatic morphology and increased lipofuscin and are perhaps smaller than expected. Among these apparently normal striatal nerve cells, there are scattered atrophic neurons that appear more darkly stained in tissue sections treated with Luxol fast blue and counterstained with hematoxylin and eosin (LHE). These atrophic neurons are referred to as neostriatal dark neurons (NDN) and have a distinct scalloped cell membrane, a granular dark cytoplasm, and an oblong nucleus with dense chromatin. NDN tend to form ill-defined clusters and are scarce in both the atrophic and the relatively spared neostriatal zones. Their density tends to increase in the intermediary zone of the neostriatum, which is flanked by the two other zones (i.e., the severely involved dorsal zone and the relatively spared ventral zone) (Vonsattel 2008; Vonsattel and DiFiglia 1998; Vonsattel et al. 1985).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







