FIGURE 12.1 The distribution of the olfactory nerves within the nose.
Within the olfactory bulbs, axons of incoming fibers synapse on dendrites of mitral and tufted cells in the olfactory glomeruli. The mitral and tufted cells are the output cells of the olfactory bulb. The axons of the second order neurons, mainly the mitral cells, course posteriorly through the olfactory tracts, which lie in the olfactory grooves, or sulci, beneath the frontal lobes in the floor of the anterior cranial fossa.
The olfactory bulbs and tracts are sometimes mistakenly called the olfactory nerves. The olfactory nerves are the unmyelinated filaments that pass through the cribriform plate. The bulbs and tracts are part of the rhinencephalon. The proximity of the olfactory tracts to the inferior surface of the frontal lobes is an important anatomic relationship (see Figure 11.3).
Olfactory information is processed in primitive areas of the brain. Olfaction is the only sensation not directly processed in the thalamus. The olfactory tracts divide into medial and lateral olfactory striae that run on either side of the anterior perforated substance. The triangular area thus formed is called the olfactory trigone. Some olfactory stria fibers decussate in the anterior commissure to join the fibers from the opposite side; some go to the olfactory trigone and tuberculum olfactorium within the anterior perforated substance. Fibers of the medial olfactory stria terminate on the medial surface of the cerebral hemisphere in the paraolfactory area, subcallosal gyrus, and inferior part of the cingulate gyrus. The lateral olfactory stria course obliquely along the anterior perforated space and beneath the temporal lobe to terminate in the uncus, anterior hippocampal gyrus, piriform cortex, entorhinal cortex, and amygdaloid nucleus (Figure 12.2). Structures collectively referred to as the primary olfactory cortex include the anterior olfactory nucleus, the piriform cortex, the anterior cortical nucleus of the amygdala, the periamygdaloid complex, and the rostral entorhinal cortex.
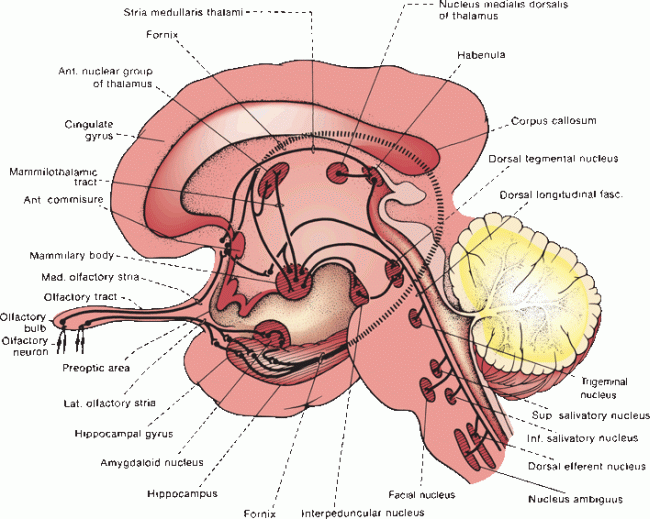
FIGURE 12.2 The olfactory pathway and its central connections.
The parahippocampal gyrus sends impulses to the hippocampus. The hippocampi and amygdaloid nuclei on the two sides are intimately related through the anterior commissure. These nuclei send projection fibers to the anterior hypothalamic nuclei, mammillary bodies, tuber cinereum, and habenular nucleus. These in turn project to the anterior nuclear group of the thalamus, interpeduncular nucleus, dorsal tegmental nucleus, striatum, cingulate gyrus, and mesencephalic reticular formation. Functional magnetic resonance imaging has shown that chemosensory signals cause activation of cortical areas not previously known to have olfactory functions. Communications with the superior and inferior salivatory nuclei are important in reflex salivation.
Olfaction is a phylogenetically ancient sensation. In lower mammals in whom olfaction is extremely important, the olfactory cortex constitutes a large part of the cerebral hemispheres. The connections between the olfactory system, hypothalamus, certain brainstem nuclei, and autonomic centers are pertinent to the understanding of many visceral functions.
The olfactory nerve is a sensory nerve with but one function—smell. The ability to perceive and identify various odors differs from person to person. Only volatile substances soluble in lipids or water are perceived as odors. In true anosmia, there is loss of ability to perceive or recognize not only scents but also flavors, for much of what is interpreted as taste involves smell. Flavor is a synthesis of sensations derived from the olfactory nerves, taste buds, and other sensory end-organs. A patient with olfactory impairment may complain of loss of taste rather than of smell. Patients with unilateral anosmia may be unaware of any impairment.
CLINICAL EXAMINATION
Impairments due to anosmia are not trivial. The problem is not merely that patients with disturbances of smell sensation miss out on some of life’s pleasures; they may also miss olfactory danger signals, such as spoiled food, smoke, and leaking gas. As with hearing, olfactory deficits are sometimes divided into (a) conductive deficits, due to processes interfering with the ability of odorants to contact the olfactory epithelium, such as nasal polyps; and (b) sensorineural or neurogenic deficits, due to dysfunction of the receptors or their central connections.
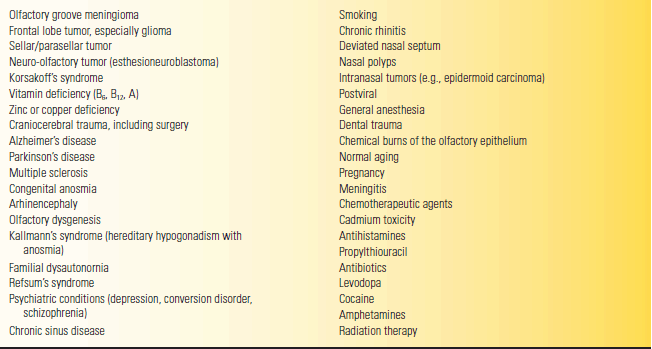
Important historical points to address in a patient with a smell or taste disturbance include past head injury; smoking; recent upper respiratory infection; systemic illness; nutrition; and exposure to toxins, medications, or illicit drugs. Feldman contends that changes in the flavor of coffee may be particularly informative. Unilateral loss of smell is more significant than bilateral, which may be caused by many conditions, primarily conductive (Table 12.1).
TABLE 12.1 Some Causes of Persistent Loss of Smell
Before evaluating smell, ensure that the nasal passages are open. Most cases of impaired smell are due to intranasal obstructions. Acute or chronic rhinitis and chronic sinusitis may seriously interfere with olfaction.
Smell is tested using nonirritating stimuli. Avoid substances such as ammonia that may stimulate the trigeminal nerve instead of the olfactory nerve, causing a response that can be confused with olfaction. The nasal passages are richly innervated by free nerve endings from the trigeminal system, which respond to many substances. Some patients with impaired taste and smell enjoy spicy food because of its stimulation of the trigeminal system.
Examine each nostril separately while occluding the other. With the patient’s eyes closed and one nostril occluded, bring the test substance near the open one. Ask the patient to sniff and indicate whether she smells something and, if so, to identify it. Repeat for the other nostril and compare the two sides. The side that might be abnormal should be examined first. Many substances can be used to test smell (e.g., wintergreen, cloves, coffee, and cinnamon). At the bedside or in the clinic, one can use mouthwash, toothpaste, alcohol, soap, and similar substances. Commercial scratch-and-sniff strips are available. Commercially available quantitative smell and taste tests include the University of Pennsylvania smell identification test (UPSIT) and the Connecticut chemosensory test. The UPSIT requires no trained personnel and may be self-administered. Its forced-choice design helps identify malingering.
The perception of odor is more important than accurate identification. Perceiving the presence of an odor indicates continuity of the olfactory pathways; identification of the odor indicates intact cortical function as well. Since there is bilateral innervation, a lesion central to the decussation of the olfactory pathways never causes loss of smell, and a lesion of the olfactory cortex does not produce anosmia. The appreciation of the presence of a smell, even without recognition, excludes anosmia.
DISORDERS OF OLFACTORY FUNCTION
Some definitions regarding disorders of smell are reviewed in Table 12.2. Loss of smell may occur in a variety of conditions (Table 12.1). Common causes of impaired smell are upper respiratory tract infection (URI), trauma, nasal and sinus disease, and normal aging. Persistent olfactory loss following a URI is the most common etiology, accounting for 15% to 25% of cases.
TABLE 12.2 Terms and Definitions Related to Olfactory Abnormalities










