The Role of Brachytherapy in the Management of Spinal Tumors
Yoshiya Yamada
ABSTRACT
Even with the most modern technology, radiation doses for spinal tumors are constrained by the relatively low tolerance of the spinal cord to the radiation. The use of brachytherapy (placing radioactive sources in very close proximity to tumors) has been described. The Inverse Square Law describes how a radiation dose is given from brachytherapy sources. As the distance increases away from the source, the dose fall-off can be described as the inverse square of the distance. Thus when brachytherapy sources are placed strategically in the tumor, the delivered dose to the nearby spinal cord can be much less. When radiation isotopes with low-energy photons, such as yttrium 90, are used, it is possible for the dose on the surface of the source to be 80% higher than a point 3 mm away. Brachytherapy in the spine is an attractive method of improving the therapeutic ratio in the radiotherapy of spine tumors by increasing the total dose delivered to a tumor without significantly increasing the dose delivered to the spinal cord or other nearby dose-sensitive structures.
INTRODUCTION
The goal of radiation therapy is to improve tumor control while minimizing the associated toxicity to normal tissues. The effects of radiation therapy on tumor and normal tissues are dependent on many factors, including the total dose of radiation, the dose per fraction, the radiosensitivity of tumor and normal tissues, and the volume of normal tissue irradiated. Of these factors, radiation dose is most likely the single most important determinant of the probability of both tumor control and normal tissue toxicity.
THE THERAPEUTIC RATIO
Radiation therapy is most effective when a high probability of tumor control and a low probability of normal tissue toxicity exist for any given dose of radiation. When these probabilities are plotted as a function of increasing radiation dose, they are typically described as S-shaped curves (Fig. 8.1). Between the low-dose area and the high-dose plateau, a region of rapid change exists, with a relatively small increase in dose. Ideally, for any given dose, the probability of toxicity should be low compared with the probability of tumor control. This difference is often referred to as the therapeutic ratio (Fig. 8.2)
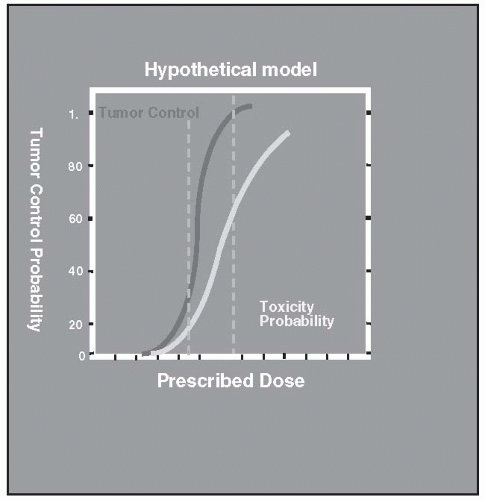 FIGURE 8.1 The therapeutic ratio is small. For a given dose, the difference between tumor control and toxicity probabilities is small. |
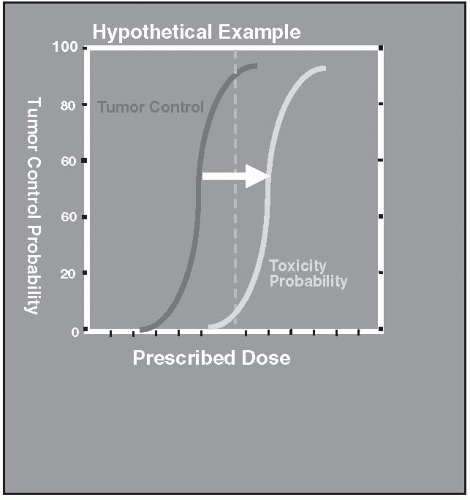 FIGURE 8.2 Idealized therapeutic ratio. The probability of tumor control is high, whereas the toxicity risk is low. |
Radiation oncologists are constantly striving to improve the therapeutic ratio. Several commonly used strategies may improve the therapeutic ratio. Altering the dose-fractionation schedule can help improve the therapeutic ratio in many instances. Increasing the dose of radiation given per fraction will increase the biologic impact of radiation therapy. Unfortunately, this phenomenon is true for both normal tissues and tumor. Conversely, reducing the dose per fraction will result in less risk of toxicity but is also more likely to spare tumor cells. When a high dose gradient can be created, in which the tumor receives a significantly higher dose than the surrounding normal tissue, the therapeutic ratio improves.
ADVANTAGES OF BRACHYTHERAPY
Brachytherapy is an ideal way of delivering high doses to the tumor while sparing normal tissues. One of the major advantages of brachytherapy in this regard is that radiation-dose intensity falls off as a function of the square of the distance (1). For example, if a radioactive seed were placed in a tumor, the dose of radiation 2 cm from the source would be one fourth of the dose at 1 cm from the source (1 cm/2 cm)2. When external-beam radiation is used, the tumor is typically 100 cm from the radiation source. Hence a minimal difference in the dose exists at 100 cm versus 101 cm (100 cm/101 cm)2. Thus the inverse-square phenomenon is much more significant when brachytherapy is used, because doses for brachytherapy are calculated with relatively short distances. Isotopes that emit very weakly penetrating photons, such as yttrium 90 (2), can also be used in brachytherapy, because the sources are placed directly in the tumor, and the photons need not travel great distances. Thus the inverse-square law coupled with low-energy brachytherapy sources allows very high doses to be delivered to the tumor while allowing relative sparing of nearby and surrounding normal tissues (Fig. 8-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







