Schizophrenia spectrum disorders in persons without infections
Schizophrenia spectrum disorder in persons with hospital contact with infections
Autoimmune disease
RRa
95 % CI
Cases
RRa
95 % CI
Cases
Reference without autoimmune disease or infection
1.00
(Reference)
29,372
1.00
(Reference)
29,372
Any autoimmune disease
1.29
1.18–1.41
483b
2.25
2.04–2.46
444b
Autoimmune diseases with suspected presence of brain-reactive antibodies:
1.48
1.31–1.68
244
2.56
2.25–2.89
243
Autoimmune hepatitis
2.75
1.38–4.83
10
8.91
6.50–11.84
43
Autoimmune thyroiditis
–
–
3
4.57
2.09–8.51
8
Celiac disease
2.11
1.09–3.61
11
2.47
1.13–4.61
8
Guillain Barre syndrome
1.22
0.58–2.19
9
2.84
1.52–4.76
12
Multiple sclerosis
1.44
1.03–1.94
39
2.10
1.37–3.06
24
Sjögren’s syndrome
2.07
0.82–4.20
6
–
–
4
Systemic lupus erythematosis
1.84
0.92–3.23
10
2.11
1.06–3.70
10
Thyrotoxicosis (Graves disease)
1.94
1.47–2.49
56
2.47
1.68–3.49
29
Type 1 diabetes
1.27
1.04–1.53
104
2.04
1.68–2.44
109
Other autoimmune diseases listed below:
1.19
1.05–1.34
256
1.95
1.70–2.23
212
Ankylosing spondylitis
1.38
0.79–2.20
15
1.68
0.72–3.25
7
Crohn’s disease
1.22
0.88–1.65
39
1.67
1.18–2.27
36
Iridocyclitis
1.32
0.87–1.91
25
1.99
1.21–3.06
18
Juvenile arthritis
1.00
0.52–1.71
11
1.77
0.95–2.97
12
Psoriasis vulgaris
1.37
1.01–1.80
47
2.77
2.07–3.63
49
Seropositive rheumatoid arthritis
0.75
0.51–1.06
28
2.15
1.52–2.95
35
Ulcerative colitis
1.22
0.97–1.51
80
1.65
1.24–2.14
52
Autoimmune diseases with too few cases to calculate the individual riskc
1.59 d
1.13–2.17
36
2.21 d
1.53–3.07
32
The Risk of Autoimmune Diseases After a Diagnosis with Schizophrenia
Individuals diagnosed with schizophrenia had a 53 % increased risk of subsequent diagnoses with autoimmune diseases, particularly the group with suspected presence of brain-reactive antibodies had a 91 % increase of risk (Table 6.2). There was a significant multiplicative interaction between having both a schizophrenia diagnosis and hospital contacts due to infections which increased the risk of subsequent autoimmune diseases by 2.7 times (Fig. 6.1) (Benros et al. 2014). In persons with schizophrenia, but no hospital contacts due to infections, the risk of autoimmune diseases was elevated with 32 % and diminished with time to a non-significant level in the time period 15 or more years after the schizophrenia diagnosis, whereas for persons with schizophrenia and infections the risk remained elevated. The increased incidence of autoimmune diseases following a diagnosis of schizophrenia might in some cases reflect symptoms of schizophrenia resulting from neuropsychiatric manifestations from the not-yet diagnosed autoimmune disease, particularly in the group with suspected presence of brain-reactive antibodies.
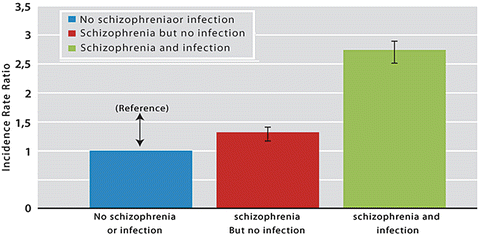
Table 6.2
Relative risk of autoimmune diseases after the diagnosis with schizophrenia spectrum disorder in Denmark (1987–2010)a
Autoimmune diseases | Cases | RR | 95 % CI | |
|---|---|---|---|---|
Persons without schizophrenia (reference) | 1.00 | (Reference) | ||
Any autoimmune disease | 142,328 | 1,401 | 1.53 | 1.46–1.62 |
Autoimmune disease with suspected presence of brain-reactive antibodies | 75,087 | 849 | 1.91 | 1.78–2.04 |
Autoimmune hepatitis | 1,878 | 40 | 3.51 | 2.51–4.73 |
Autoimmune thyroiditis | 3,386 | 23 | 0.90 | 0.58–1.33 |
Celiac disease | 2,350 | 20 | 1.33 | 0.82–2.03 |
Guillain Barre syndrome | 1,648 | 24 | 2.73 | 1.77–3.99 |
Multiple sclerosis | 9,759 | 83 | 1.57 | 1.29–1.90 |
Sjögren’s syndrome | 1,994 | 19 | 1.31 | 0.80–2.00 |
Systemic lupus erythematosis | 2,101 | 19 | 1.57 | 0.96–2.39 |
Thyrotoxicosis | 17,308 | 136 | 1.10 | 0.93–1.30 |
Type 1 diabetes | 28,272 | 478 | 2.83 | 2.58–3.10 |
Other autoimmune diseases | 80,979 | 642 | 1.21 | 1.11–1.30 |
Alopecia areata | 1,377 | 9 | 1.05 | 0.50–1.90 |
Ankylosing spondylitis | 3,661 | 21 | 0.78 | 0.49–1.16 |
Crohn’s disease | 12,117 | 98 | 1.33 | 1.08–1.61 |
Idiopathic thrombocytopenic purpura | 1,660 | 14 | 1.36 | 0.76–2.21 |
Iridocyclitis | 9,220 | 80 | 1.18 | 0.94–1.46 |
Pernicious anemia | 1,082 | 19 | 2.59 | 1.58–3.95 |
Polymyalgia rheumatica | 2,396 | 11 | 0.61 | 0.31–1.04 |
Primary adrenocortical insufficiency | 785 | 20 | 3.81 | 2.36–5.79 |
Primary biliary cirrhosis | 478 | 9 | 2.65 | 1.26–4.81 |
Psoriasis vulgaris | 13,269 | 190 | 2.13 | 1.84–2.45 |
Seropositive rheumatoid arthritis | 15,768 | 82 | 0.75 | 0.60–0.93 |
Ulcerative colitis | 22,289 | 146 | 0.99 | 0.84–1.16 |
Autoimmune diseases with too few cases to calculate the individual riskb, c | 6,701 | 25 | 0.72 | 0.47–1.04 |

Fig. 6.1
Relative risk of subsequent autoimmune diseases in people with schizophrenia (significant multiplicative interaction between schizophrenia and infection on the risk autoimmune diseases (p = 0.004). Source: Benros et al. Am J Psychiatry 2014
Associations with a Family History of Either Autoimmune Diseases or Schizophrenia
A family history with autoimmune diseases has been shown to increase the risk of schizophrenia by 10 % and a family history with schizophrenia increases the risk of autoimmune diseases by 6 % (Benros et al. 2014; Eaton et al. 2010). However, a family history with bipolar disorder was not significantly associated with autoimmune diseases, and there was no association in the reverse direction either (Benros et al. 2014; Eaton et al. 2010). A family history with the following specific autoimmune diseases has been associated with an increased incidence of schizophrenia in a nationwide Danish study (Eaton et al. 2010): autoimmune hepatitis, type 1 diabetes, Sjögrens syndrome, iridocyclitis, multiple sclerosis, psoriasis vulgaris, and dermatopolymyositis, whereas only a family history with pernicious anemia was associated with bipolar disorder out of the 30 autoimmune diseases studied. The association with a family history of diabetes type 1 and autoimmune thyrotoxicosis with schizophrenia has been confirmed in other populations as well (Wright et al. 1996; Gilvarry et al. 1996). A family history of schizophrenia was associated with pernicious anemia, diabetes type 1, iridocyclitis, autoimmune hepatitis, systemic lupus erythematosus, Sjögren’s syndrome, and primary biliary cirrhosis.
Associations Between Autoimmune Diseases and Depression
Several autoimmune diseases, such as diabetes type 1, multiple sclerosis, systemic lupus erythematosus, and autoimmune thyroid disease, have been associated with depression in smaller studies (Korczak et al. 2011; Strous and Shoenfeld 2007; Gold and Irwin 2009; Padmos et al. 2004; Pop et al. 1998; Vonk et al. 2007). Rheumatoid arthritis has been associated with depression in several studies and in a meta-analysis (Dickens et al. 2002). A Danish nationwide study on 91,637 cases with a first-time hospital contact due to mood disorders showed that a prior hospital contact because of autoimmune diseases increased the risk of a subsequent mood disorder diagnosis by 57 %, but when separating the effect of infections, autoimmune diseases were associated with an increased risk by 45 % compared to the general population (Table 6.3) (Benros et al. 2013). The risk of developing mood disorders was elevated to the greatest degree in the group of autoimmune diseases with suspected presence of brain-reactive antibodies (58 %), particularly when combined with an infection (2.49 times increase of risk). In another population-based study (Eaton et al. 2010), a 70 % increased risk was found of developing bipolar disorder within 4 years of an autoimmune disease diagnosis and a 20 % increased risk in the time span from 5 years and onwards after the diagnosis, compared to the background population.
Table 6.3
Relative risk of mood disorders with a hospital contact in persons with hospital contact for autoimmune diseases and infections in Denmark (1977–2010)a
Mood disorders in persons without infections | Mood disorder in persons with infections | |||||
|---|---|---|---|---|---|---|
Autoimmune disease | Relative riskb | 95 % CI | Case patients | Relative riskb | 95 % CI | Case patients |
Persons without autoimmune disease (reference) | 1.00 | (Reference) | 60,361 | 1.62 | 1.60–1.64 | 27,081 |
Any autoimmune disease | 1.45 | 1.39–1.52 | 2,082c | 2.35 | 2.25–2.46 | 2,113c |
Autoimmune diseases with suspected presence of brain-reactive antibodies: | 1.58 | 1.49–1.68 | 1,057 | 2.49 | 2.35–2.65 | 1,123 |
Autoimmune hepatitis | 2.28 | 1.53–3.41 | 24 | 3.13 | 2.39–4.11 | 52 |
Autoimmune thyroiditis | 1.05 | 0.72–1.52 | 28 | 1.63 | 1.09–2.43 | 24 |
Celiac disease | 1.91 | 1.41–2.60 | 41 | 1.90 | 1.32–2.73 | 29 |
Guillain Barre syndrome | 1.61 | 1.14–2.26 | 33 | 2.24 | 1.58–3.17 | 32 |
Multiple sclerosis | 1.52 | 1.30–1.77 | 162 | 2.42 | 2.06–2.86 | 142 |
Sjögren’s syndrome | 1.79 | 1.23–2.61 | 27 | 2.58 | 1.79–3.71 | 29 |
Systemic lupus erythematosis | 2.16 | 1.61–2.89 | 45 | 2.19 | 1.65–2.92 | 47 |
Thyrotoxicosis (Graves disease) | 1.28 | 1.12–1.45 | 228 | 1.90 | 1.63–2.21 | 165 |
Type 1 diabetes | 1.77 | 1.61–1.94 | 469 | 2.84 | 2.62–3.07 | 603 |
Other autoimmune diseases listed below: | 1.35 | 1.27–1.43 | 1,206 | 2.22 | 2.08–2.36 | 1,282 |
Ankylosing spondylitis | 1.23 | 0.91–1.65 | 44 | 2.02 | 1.46–2.81 | 36 |
Crohn’s disease | 1.75 | 1.52–2.01 | 191 | 2.32 | 2.04–2.65 | 224 |
Iridocyclitis | 1.22 | 1.00–1.48 | 101 | 2.08 | 1.70–2.54 | 94 |
Juvenile arthritis | 1.20 | 0.88–1.64 | 39 | 2.48 | 1.93–3.19 | 61 |
Psoriasis vulgaris | 1.58 | 1.38–1.81 | 203 | 2.60 | 2.27–2.97 | 212 |
Seropositive rheumatoid arthritis | 1.08 | 0.93–1.25 | 167 | 2.18 | 1.89–2.52 | 183 |
Ulcerative colitis | 1.41 | 1.27–1.57 | 331 | 2.27 | 2.04–2.54 | 315 |
Alopecia areata | 1.08 | 0.66–1.77 | 16 | 2.43 | 1.60–3.69 | 22 |
Autoimmune hemolytic anemia | 2.53 | 1.27–5.06 | 8 | 2.28 | 1.02–5.07 | 6 |
Dermatopolymyositis | 1.25 | 0.60–2.62 | 7 | 3.40 | 2.01–5.74 | 14 |
Idiopathic thrombocytopenic purpura | 1.18 | 0.71–1.96 | 15 | 2.13 | 1.37–3.30 | 20 |
Myasthenia gravis | 1.19 | 0.62–2.30 | 9 | – | – | 4 |
Pernicious anemia | 1.37 | 0.81–2.30 | 14 | 2.14 | 1.24–3.68 | 13 |
Primary adrenocortical insufficiency | 2.58 | 1.53–4.35 | 14 | 1.64 | 0.95–2.82 | 13 |
Primary biliary cirrhosis | 1.74 | 0.78–3.88 | 6 | – | – | 3 |
Pemphigus | – | – | 2 | 4.31 | 1.94–9.60 | 6 |
Pemphigoid | – | – | 1 | – | – | 1 |
Polymyalgia rheumatica | 1.30 | 0.77–2.19 | 14 | 3.81 | 2.53–5.73 | 23 |
Scleroderma | 1.03 | 0.56–1.92 | 10 | 3.18 | 2.05–4.93 | 20 |
Vitiligo | 1.24 | 0.70–2.17 | 12 | 2.01 | 1.14–3.54 | 12 |
Wegener’s granulomatosis | – | – | 2 | 2.37 | 1.18–4.75 | 8 |
Associations Between Infections and Schizophrenia
Epidemiological studies have indicated a dose–response relationship between urbanicity during upbringing and the risk of schizophrenia (Pedersen and Mortensen 2001), which could be related to, for instance, an increased probability of acquiring infections in urban environments (Yolken and Torrey 2008). An increased risk of schizophrenia has been associated with many different infectious agents, and a recent meta-analysis displayed significant associations between schizophrenia with Toxoplasma gondii, human herpesvirus 2, Borna disease virus, human endogenous retrovirus W, Chlamydophila psittaci, and Chlamydophila pneumonia (Arias et al. 2012). T. gondii infection has in many studies been associated with schizophrenia (Yolken and Torrey 2008; Niebuhr et al. 2008a; Torrey et al. 2007), and a recent population-based study indicated a dose–response relationship correlating with the serum titer of toxoplasma and the subsequent risk of schizophrenia (Pedersen et al. 2011). An increased risk of schizophrenia has also been associated with herpes simplex virus infection, detected by both serum antibodies (Dickerson et al. 2003; Niebuhr et al. 2008b) and CSF antibodies (Bartova et al. 1987). Cytomegalovirus (CMV) antibody titers have been found to be higher in the serum of patients with schizophrenia (Torrey et al. 2006; Leweke et al. 2004) and in the CSF (Torrey et al. 1982). However, studies have shown contradictory associations between CMV and schizophrenia, with stronger associations in newly diagnosed and untreated patients (Leweke et al. 2004), and to date, no neuropathologic evidence of CMV in the brains of patients with schizophrenia has been found (Torrey et al. 2006). Retroviral antigens and products have been identified in patients with schizophrenia (Karlsson et al. 2001; Hart et al. 1999), and other viruses associated with schizophrenia include Borna virus, where an increased serum prevalence having been observed (Chen et al. 1999). An increased prevalence of Chlamydophila infection has also been observed in patients with schizophrenia, especially when linked to genetic markers of the immune system (Fellerhoff et al. 2007). Additionally, post-mortem studies have found increased prevalence of Chlamydophila DNA in brains from patients with schizophrenia (Fellerhoff and Wank 2011). Psychotic disorders have also in population-based studies been associated with higher rates of several infections, such as pneumonia and pneumococcal disease (Crump et al. 2013a, b; Smith et al. 2013; Seminog and Goldacre 2013).
The Risk of Schizophrenia After Infections
A large-scale Danish nationwide study on 39,076 persons with a diagnosis of schizophrenia spectrum disorders showed that any history of hospitalization with infection increased the risk of schizophrenia by 60 % (Benros et al. 2011). The risk of schizophrenia increased in a significant dose–response relationship with the number of infections (Fig. 6.2) and were increased the most with the temporal proximity of the last infection. The results remained significant after excluding persons diagnosed with substance use disorders, and there were no important differences in the relative risk added in persons with or without a psychiatric family history. Hospital contact due to infection had previously occurred in 23.6 % of persons diagnosed with schizophrenia spectrum disorders, yielded a population-attributable risk of 9 % associated with hospital contacts due to infections. A subsequent nationwide studies from Sweden confirmed the associations with previous hospital contacts with infections and schizophrenia spectrum disorders (Blomstrom et al. 2014). A subsequent Danish nationwide study on a narrower cohort during a period with complete follow-up of all hospital contacts showed that 45 % of persons with schizophrenia had a previous hospital contact with infection, which increased the risk of schizophrenia by 41 %, with bacterial infections increasing the risk by 63 % (Nielsen et al. 2014).
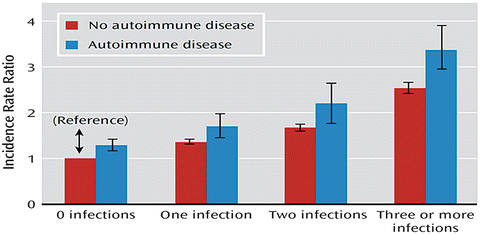

Fig. 6.2
Relative risk of schizophrenia spectrum disorders in persons with autoimmune disease and infections. Source: Benros et al. Am J Psychiatry. 2011
A recent meta-analysis has reported significant associations between childhood CNS infections and schizophrenia (Khandaker et al. 2012). Most studies on individuals with hospitalizations for CNS infections have found an increased risk of schizophrenia, including population-based studies from Denmark, Sweden, Finland, and Australia (Dalman et al. 2008; Benros et al. 2011; Liang and Chikritzhs 2012; Abrahao et al. 2005; Koponen et al. 2004). However, some studies did not find a significant association with CNS infections (Weiser et al. 2010; Suvisaari et al. 2003). In nationwide studies a broad spectrum of infections have been associated with schizophrenia, where sepsis and hepatitis infections were associated with the most elevated risk (2 and 4.9 times, respectively Table 6.4).
Table 6.4
Relative risk of schizophrenia spectrum diagnosis in persons with autoimmune diseases depending on the site of infection, Denmark, 1977–2006
Only infection (no autoimmune disease) | Autoimmune disease | |||||
|---|---|---|---|---|---|---|
Site of infection | IRR | 95 % CI | Cases | IRR | 95 % CI | Cases |
Sepsis infections | 1.95 | 1.47–2.51 | 55 | 4.98 | 2.49–8.73 | 10 |
Hepatitis infections | 4.89 | 4.26–5.58 | 212 | 8.89 | 6.03–12.53 | 29 |
Gastrointestinal infections | 1.32 | 1.26–1.39 | 1,847 | 1.82 | 1.46–2.24 | 83 |
Skin infection | 1.71 | 1.62–1.80 | 1,427 | 2.14 | 1.69–2.66 | 74 |
Pregnancy related infection | 1.14 | 0.98–1.31 | 185 | 1.22 | 0.48–2.47 | 6 |
Respiratory infections | 1.53
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||


