Front Neurol Neurosci. Basel, Karger, 2014, vol 33, pp 147-161 (DOI: 10.1159/000351915)
______________________
Antithrombotic Therapy in Transient Ischemic Attack Patients
V.E. Held · M.E. Wolf · M.G. Hennerici
Department of Neurology, UniversitätsMedizin Mannheim, University of Heidelberg, Mannheim, Germany
______________________
Abstract
Historically, studies of antithrombotic therapy in ischemic cerebrovascular disease have included both stroke and transient ischemic attack (TIA). Thus, therapy regimes are very similar. Aspirin (75- 325 mg within 48 h after onset of symptoms) is still the standard antithrombotic treatment because other agents have performed similarly (or worse). Combinations of agents have shown mixed results. Aspirin combined with clopidogrel has failed to show a significant reduction of stroke/TIA recurrences but increased the bleeding risk if taken for more than several months. The combination of aspirin and dipyridamole is slightly better than aspirin alone and in particular reduced nonfatal stroke/TIA – hence it is recommended as an alternative and may be used in patients with recurrent events while on regular aspirin. In contrast, combined treatment is regularly recommended after endovascular interventions and if both cardio- and cerebrovascular diseases are present. Warfarin and similar compounds have long been the standard treatment for most patients with permanent, paroxysmal or intermittent non-valvular atrial fibrillation, for which there is excellent evidence in most patients (CHADS-VASc score >1). New compounds have been approved in recent years and shown to reduce either ischemic events, intracranial bleeding complications or both when compared with warfarin. None of them requires regular therapy monitoring. Because there are no head-to-head comparisons of these newer agents, definite recommendations as to which to choose, and when, are hard to make. However, there are some notable differences as well as new approved entities.
Copyright © 2014 S. Karger AG, Basel
Antiplatelet Therapy
Monotherapy
Aspirin
Aspirin has been studied in primary and secondary prevention of stroke and other vascular events in doses ranging from as low as 30 to as high as 1,500 mg per day. From a pharmacological standpoint, 75 mg per day is enough to block the production of the prothrombotic thromboxane A2 by inhibiting the enzyme cyclooxygenase virtually completely (see fig. 1). Doses below 50 mg per day make for a less complete blockade but also preserve the production of the antithrombotic prostacyclin. The Antiplatelet Trialists’ Collaboration has been performing large meta-analyses of different antiplatelet drugs. In trials comparing different doses of aspirin, ≥75 mg per day was not significantly superior or inferior to <75 mg per day. In studies examining aspirin versus placebo, doses below 75 mg per day were slightly less effective. There was a trend towards the 75-150 mg per day range being most effective in preventing vascular events. In a subset of 21 studies of patients with previous transient ischemic attack (TIA) or stroke, the Antiplatelet Trialists’ Collaboration concluded that aspirin reduced the risk of stroke, myocardial infarction, or vascular death from 21.4 to 17.8% over a 2-year period, with an odds reduction of 22% [1]. Risk reductions were similar for men and women, across age groups, and in the presence or absence of diabetes mellitus or elevated blood pressure.
However, aspirin increases the risk of major bleeding by about 70% over placebo, but the absolute annual risk increase is only 0.13%, meaning that about 770 patients have to take aspirin for one year to induce one additional major bleeding incident [2]. Doses >325 mg per day increase this risk, and hence recommended doses are 50-325 mg per day. Because a few days of treatment are required to reach a steady state, loading with doses of 160-300 mg per day is advised. Aspirin cannot be recommended for primary prevention in the general population.
Conditions that may reduce the effectiveness of aspirin include polymorphisms in the gene for cyclooxygenase 1 or other enzymes involved in thromboxane production, increased production of thromboxane in cells other than platelets, high platelet turnover, and concomitant use with other drugs, especially NSAIDS such as ibuprofen and naproxen, which compete for the active center of cyclooxygenase. While poor response to aspirin is related to an increased risk of vascular events and may be overcome by increasing the dose or switching to another agent, there is no direct evidence to support this approach.
Ticlopidine
Ticlopidine is an irreversible inhibitor of the P2Y 12 receptor. Via this mechanism, it blocks platelet activation through ADP and the glycoprotein IIb/IIIa pathway (see fig. 1). It has been shown to be about as effective in preventing cardiovascular events as aspirin. Because of the preferable safety profile, it has been largely abandoned for its successor, clopidogrel.
Clopidogrel
The standard dose for clopidogrel is 75 mg per day; loading with 300 mg is advised. Most of the evidence on the use of clopidogrel in secondary prevention of vascular events comes from the 1996 CAPRIE trial. Qualifying events for inclusion were a mix of atherosclerotic conditions, such as recent ischemic stroke, myocardial infarction, peripheral artery disease, but not TIA. For all these groups, clopidogrel led to an 8.7% (0.3-16.5) relative risk reduction for a combined end point of ischemic stroke, myocardial infarction, or vascular death compared to aspirin. This treatment effect was, however, not the same among patient groups with similar diseases. While the effect was large and highly significant in the peripheral artery disease group, effects were small and insignificant in patients with ischemic stroke or myocardial infarction as the qualifying events. The efficacy is usually presumed to be similar in TIA and stroke, on the rationale that the two share the same pathophysiology. Bleeding rates were comparable or slightly smaller for clopidogrel [3].
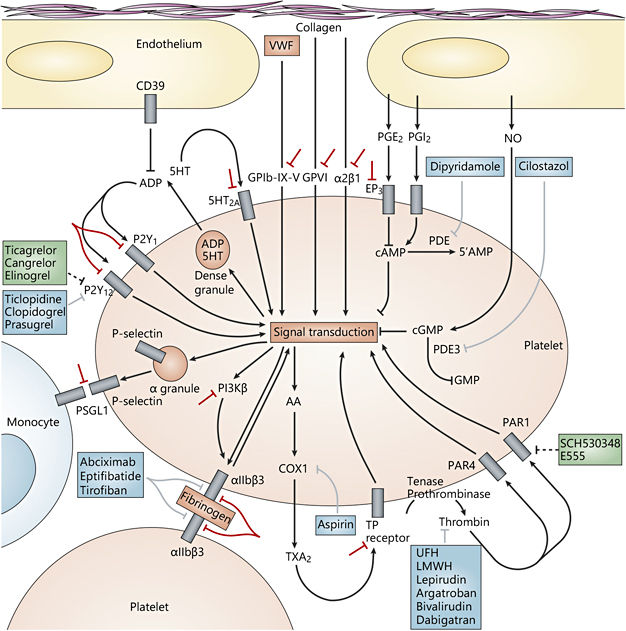
Fig. 1. The complex pathways of platelet activation and molecular targets for antiplatelet agents. Antiplatelet drugs are shown in blue, drugs in clinical development or recently approved are shown in green, investigational strategies are shown in red. AA = Arachidonic acid; COX1 = cyclooxygenase 1; EP 3 = prostaglandin E2 receptor EP 3 subtype; GPVI = glycoprotein VI; LMWH = low-molecularweight heparin; NO = nitric oxide; PAR = proteinase-activated receptor; PDE = phosphodiesterase; PG = prostaglandin; PI3Kβ = phosphoinositide 3-kinase β-isoform; PSGL1 = P-selectin glycoprotein ligand 1; TP = thromboxane prostanoid; TXA2 = thromboxane A2; UFH = unfractionated heparin; VWF = Von Willebrand factor. Reprinted with permission from Macmillan Publishers Ltd [24].
Conditions that may decrease the effect of clopidogrel mainly include concomitant therapy with other drugs or genetic reasons. Clopidogrel is a prodrug, which is transformed to its active metabolite by the cytochrome P system, most notably CYP2C19, CYP3A4/5, CYP2B6, and CYP2C9. Inhibitors of these enzymes may thus decrease the effect of clopidogrel. One such drug is omeprazole (and to a lesser degree other proton pump inhibitors) and should be avoided in these patients. Also, several polymorphisms in the genes to these CYP enzymes have been described, which lead to decreased clopidogrel activation.
Cilostazol
Cilostazol is an agent with a similar mode of action as dipyridamole (see fig. 1). It has been studied in secondary prevention after stroke in 2 RCTs (the CASISP and CSPS II trials), where it was more effective than aspirin in preventing vascular events (RR 0.72, 95% CI: 0.57-0.91) with a lower risk of hemorrhagic stroke (RR 0.26, 95% CI: 0.13-0.55) [4]. The caveat here is that both trials included virtually only Asians who have a higher risk of hemorrhagic stroke and hemorrhagic transformation of ischemic stroke than Caucasians or people of African descent. Transferability of study results may be limited and the drug has not been approved in Europe.
Terutroban
Terutroban is a selective antagonist of the thromboxane A2 receptor, which was said to share anti-inflammatory and antithrombotic effects in addition to antiplatelet activities. The largest secondary prevention trial after stroke/TIAs in patients with atherothrombotic small and large artery diseases, the PERFORM trial, was prematurely stopped for futility reasons >36 months after recruitment of patients. Terutroban was comparable to aspirin in terms of safety and effectiveness.
Other Agents
Prasugrel and ticagrelor are, like ticlopidine and clopidogrel, inhibitors of the P2Y 12 receptor (see fig. 1). While they have been approved in patients with different coronary conditions, they have not been studied for secondary prevention after stroke or TIA.
Tirofiban is an inhibitor of the glycoprotein GPIIbIIIA (αIIBβ 3), an important molecule in platelet activation (see fig. 1). Since it has to be given IV, it is not suitable for a long-term therapy in secondary prevention. It has been proposed for use in patients shortly after acute ischemic stroke, especially when they are thought to be at high risk of ischemic stroke progression, such as in patients with stenosis of the basilar artery and in low-flow territories. The phase II SaTIS trial has shown that such an approach is feasible and safe with low rates of hemorrhagic complications, but with a lower 5-month mortality rate in the tirofiban group (OR 4.05, 95% CI: 1.1-14.9) [5]. Unfortunately, a phase III trial with this drug has not been performed.
Abciximab is a monoclonal antibody of GPIIbIIIA. The AbESTT-II trial comparing abciximab and placebo in acute stroke was stopped prematurely because, at 3 months, while mortality and morbidity were similar in the study groups, the bleeding risk was excessively higher in the abciximab group.
Combination Regimens
Aspirin plus Extended-Release Dipyridamole
Dipyridamole has a dual mode of action. It blocks adenosine reuptake and thus leads to vasodilation. This effect is temporary because adenosine receptors are consequently downregulated. Its second mode of action is by blocking phosphodiesterase in platelets, which leads to decreased platelet aggregation. Dipyridamole has been shown to be about as effective as aspirin in preventing vascular events [6]. The ESPS2 and ESPRIT trials gave an indication that the combination of an extendedrelease formula of dipyridamole and aspirin (usually 200/25 b.i.d.) reduces the risk of vascular events over aspirin alone after stroke or TIA (HR 0.82, 95% CI: 0.74- 0.91) [7], but the subsequent large PRoFESS trial failed to confirm superiority over clopidogrel [8]. Since clopidogrel is no more effective than aspirin in preventing recurrent stroke, the question was raised whether the effects seen in these earlier trials were due to the relatively low doses given in the aspirin alone groups (50 mg per day in the ESPS2 trial, 30-325 mg per day in ESPRIT, with 44% receiving only 30 mg per day). More recently, the JASAP trial used an aspirin dose of 81 mg per day, closer to the doses commonly prescribed. This trial failed to show equality of aspirin plus dipyridamole compared to aspirin alone [9]. This might have been due to unexpectedly low event rates and short study duration, but there is no evidence that suggests that results would have differed had the study been larger or longer. In PRoFESS, the bleeding risk was slightly higher for aspirin plus dipyridamole than for clopidogrel (HR 1.15, 95% CI: 1.00-1.32) [8].
Dipyridamole is a vasodilator and may cause headaches in up to 30% of patients, usually within the first days, and up to 10% of patients will discontinue the drug because of this. Due to the lower aspirin dose of 50 mg per day compared to the aspirin doses usually prescribed to patients with coronary disease, its use is sometimes discouraged in these patients.
Aspirin plus Clopidogrel
The combination of aspirin and clopidogrel has been of especial interest. It is widely used in patients with acute coronary syndrome or after stenting. Two large trials studied this combination in secondary prevention after stoke or TIA: CHARISMA and MATCH. The CHARISMA trial compared this combination versus aspirin alone in a broad group at high risk for vascular events due to different conditions (about 25 and 12% had a history of stroke and TIA, respectively) and found only minor, mostly insignificant differences in efficacy and safety [10]. The MATCH trial compared the combination versus clopidogrel alone after recent ischemic stroke or TIA [11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








