Tourette Syndrome and Tic Disorders
DSM-IV-TR Diagnostic Criteria
Tourette’s Disorder
Both multiple motor and one or more vocal tics have been present at some time during the illness, although not necessarily concurrently. (A tic is a sudden, rapid, recurrent, nonrhythmic, stereotyped motor movement or vocalization.)
The tics occur many times a day (usually in bouts) nearly every day or intermittently throughout a period of more than 1 year, and during this period there was never a tic-free period of more than 3 consecutive months.
The onset is before age 18 years.
The disturbance is not due to the direct physiological effects of a substance (e.g., stimulants) or a general medical condition (e.g., Huntington’s disease or postviral encephalitis).
Chronic Motor or Vocal Tic Disorder
Single or multiple motor or vocal tics (i.e., sudden, rapid, recurrent, nonrhythmic, stereotyped motor movements or vocalizations), but not both, have been present at some time during the illness.
The tics occur many times a day nearly every day or intermittently throughout a period of more than 1 year, and during this period there was never a tic-free period of more than 3 consecutive months.
The onset is before age 18 years.
The disturbance is not due to the direct physiological effects of a substance (e.g., stimulants) or a general medical condition (e.g., Huntington’s disease or postviral encephalitis).
Criteria have never been met for Tourettes Disorder.
Transient Tic Disorder
Single or multiple motor and/or vocal tics (i.e., sudden, rapid, recurrent, nonrhythmic, stereotyped motor movements or vocalizations)
The tics occur many times a day, nearly every day for at least 4 weeks, but for no longer than 12 consecutive months.
The onset is before age 18 years.
The disturbance is not due to the direct physiological effects of a substance (e.g., stimulants) or a general medical condition (e.g., Huntington’s disease or postviral encephalitis).
Criteria have never been met for Tourettes Disorder or Chronic Motor or Vocal Tic Disorder.
Tic Disorder Not Otherwise Specified
This category is for disorders characterized by tics that do not meet criteria for a specific Tic Disorder. Examples include tics lasting less than 4 weeks or tics with an onset after age 18 years.
(Adapted with permission from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
Transient tic behaviors are commonplace among school-age children. Estimates range between 4% and 24% of school-age children experiencing tics. The number of children experiencing chronic motor tic disorders is roughly one-quarter this number. Once thought to be much rarer, the current lifetime prevalence estimates for TS ranges from 0.1% to 1%.
The prevalence of tic disorders peaks during the late first and early second decades of life due to the clinical course of the disorder. They are roughly one-third as prevalent in adulthood. Boys are about twice as likely to be affected by tic disorders as girls.
The current understanding of Tourette syndrome (TS) provides a good working model for understanding the pathogenesis of other childhood neuropsychiatric disorders: a genetically determined vulnerability, age-dependent expression of symptoms reflecting maturational factors, sexual dimorphism, stress-dependent fluctuations in symptom severity, and environmental influences on the phenotypical expression of the tic.
Strong evidence implicates the basal ganglia and corticostriatal−thalamocortical (CSTC) abnormalities as central to the pathogenesis of tics. Indirect evidence for the involvement of the basal ganglia in the pathogenesis of TS comes from the association with basal ganglia pathology of other movement disorders such as Sydenham’s chorea, Huntington’s disease, hemiballismus and Parkinson’s disease. Direct evidence supporting CSTC abnormalities in TS comes from neuroimaging, neuropathological and neurosurgical studies.
CSTC loops are multiple, parallel, but partially overlapping, neuroanatomical circuits, relaying information from most regions of the cortex to subcortical areas, particularly the striatum and thalamus, which in turn project to a subset of the original cortical areas. In CSTC loops, the basal ganglia can be viewed as a way station between intention and action (thought, affect and movement). Tics, repetitive and stereotyped movements are believed to arise from imbalances in focal populations in the basal ganglia circuits. A tic may arise through failure of inhibition of a discrete population of striatal projection neurons which become abnormally active and cause an unwanted disinhibition of a corresponding group of thalamocortical projection neurons. Thus a cortical motor pattern generator is activated so that unintended urges and motor and vocal tics are triggered.
Altered dopaminergic functioning has also been strongly implicated in the pathogenesis of TS. Evidence for abnormal dopamine neurotransmission in TS is inferred from two clinical observations. First, blockade of dopamine receptors by neuroleptic drugs suppresses tics in a majority of patients. In addition, dopamine-releasing drugs such as cocaine and amphetamines have been reported to precipitate or exacerbate tics. It has been shown that TS patients release more dopamine at dopaminergic synapses in response to amphetamine. Second, the importance of dopamine in TS is supported by brain imaging using dopamine ligands in single photon emission computed tomography and positron emission tomography. In one twin study, tic severity was related to dopamine D2 receptor binding in the head of the caudate nucleus.
Although the particular genetic factors responsible for TS remain largely undiscovered, there is a sizable heritable component responsible for this illness. Early twin studies have suggested that the concordance rate for TS among monozygotic twin pairs is greater than 50% while the concordance of dizygotic twin pairs is about 10%. If twins with chronic motor tic disorder are included, concordance figures increase to 77% for monozygotic and 30% for dizygotic twin pairs. Differences in the concordance of monozygotic and dizygotic twin pairs indicate that genetic factors play an important role in the etiology of TS and related conditions. These figures also suggest that nongenetic factors are critical in determining the nature and severity of the clinical syndrome.
The overall risk that an offspring of a parent with TS will develop TS is approximately 10–15%, while the risk of offspring developing a tic disorder (20–29%) or obsessive–compulsive disorder (OCD) (12–32%) is slightly higher. The risk of developing tic disorders in male offspring is higher than for females, while the risk of developing OCD is lower.
Recently, an analysis of a chromosomal anomaly in one TS patient led to the identification of two sequence variants in the SLITRK1 gene in approximately 1% of TS cases. Specifically, among 174 unrelated probands with TS, one frameshift mutation and two identical variants in the 3′ untranscribed region of this gene were identified. None of these anomalies was demonstrated in over 3,600 controls. SLITRK1 mRNA is expressed in the human fetal brain at 20 weeks gestation in multiple neuroanatomical areas implicated in TS neuropathology including the cortical plate, striatum, globus pallidus, thalamus and subthalamic nucleus. While other genes of major effect are likely to be found, it is becoming ever clear that TS is likely to be a polygenetic disorder with complex gene–environment interactions that have yet to be fully elucidated.
The existence of monozygotic twins discordant for tic severity emphasizes the importance of environmental factors in the phenotypic expression of TS. In monozygotic twins discordant for tic severity, the twin with more severe tics has usually had the lower birth weight and has a greater number of dopamine receptor sites in the caudate nucleus. In observational and case control studies, low (Activity, Pulse, Grimace, Appearance, and Respiration); APGAR scores, stressful maternal life circumstances during pregnancy and maternal first-trimester nausea are risk factors for TS.
Tic disorders have long been identified as “stress-sensitive” conditions. Typically, symptom exacerbations follow in the wake of stressful life events. These events need not be adverse in character. Clinical experience suggests that in some unfortunate instances a vicious cycle can be initiated in which tic symptoms are misunderstood by the family and teachers, leading to active attempts to suppress the symptoms by punishment and humiliation. These efforts can lead to a further exacerbation of symptoms and an increase in stress in the child’s interpersonal environment. Prospective longitudinal studies have begun to examine systematically the effect of intramorbid stress. These studies indicate that patients with TS experience more stress than matched healthy controls and that antecedent stress may play a role in subsequent tic exacerbations.
Post-infectious autoimmune mechanisms have been implicated in a small number of post-streptococcal cases of TS and OCD known as Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal (PANDAS) infections. PANDAS cases are characterized by a sudden onset of symptoms, episodic course, and abrupt symptom exacerbations, sometimes accompanied by adventitious movements. The most salient differentiating factor between PANDAS and other cases of TS and OCD is an exacerbation of OCD and tic symptoms accompanied by positive streptococcal throat cultures, increased antistreptoccal and antineuronal antibodies, but no evidence of rheumatic fever. Evidence that PANDAS and post-streptococcal autoimmune factors are associated with some cases of tics and OCD comes from a variety of sources. Sydenham’s chorea, a late autoimmune sequela of rheumatic fever, is often accompanied by tics, mood changes, OCD, attention-deficit hyperactivity disorder (ADHD) and anxiety. There is a large series of over 50 children in whom exacerbations of OCD or tics were accompanied by positive streptococcal throat cultures and increased antistreptoccal and antineuronal antibodies without evidence of rheumatic fever. One case-control study found an increased proportion of group A β-hemolytic streptococcal (GABHS) infections (odds ratio = 3.05) within the preceding 3 months in children newly-diagnosed with TS compared to well-matched controls. This suggests a role for post-streptococcal autoimmune factors in a large proportion of cases. A larger odds ratio (12.1) was demonstrated when subjects had multiple GABHS infections in the previous year, suggesting a dose-dependent effect of exposure to infection. However, prospective longitudinal studies have failed to provide evidence that newly acquired GABHS infections precede tic exacerbations. The relative prevalence of PANDAS cases and the importance of autoimmune mechanisms to TS are controversial.
Tics are sudden, repetitive movements, gestures, or utterances that typically mimic an aspect of normal behavior. Individual tics rarely last more than a second. Many tics occur in bouts with brief inter-tic intervals of less than one second. Individual tics can occur singly or together in an orchestrated pattern. They vary in intensity or forcefulness. Motor tics, which can be viewed as disinhibited fragments of normal movement, can vary from simple, abrupt movements such as eye blinking, nose twitching, head or arm jerking, or shoulder shrugging to more complex movements that appear to have a purpose, such as facial or hand gestures or sustained looks. These two phenotypic extremes of motor tics are classified as simple and complex motor tics respectively. Similarly, phonic tics can be classified into simple and complex. Simple vocal tics are sudden, meaningless sounds such as throat clearing, coughing, sniffing, spitting, or grunting. Complex phonic tics are more protracted, meaningful utterances, which vary from prolonged throat clearing to syllables, words or phrases and to even more complex behaviors such as repeating ones own words (palalalia) or those of others (echolalia) and, in rare cases, the use of obscenities (coprolalia).
The severity of tics in TS waxes and wanes throughout the course of the disorder. The tics of TS and other tic disorders are highly variable from minute-to-minute, hour-to-hour, day-to-day, week-to-week, month-to-month, and even year-to-year. Tic episodes occur in bouts, which in turn also tend to cluster. Tic symptoms, however, can be exacerbated by stress, fatigue, extremes of temperature and external stimuli (e.g., in echolalia). Intentional movement attenuates tics in the affected area and intense involvement in activities tends to dissipate tic symptoms.
Many individuals with tics, especially those post-pubertal, are aware of premonitory urges: feelings of tightness, tension, or itching that are accompanied by a mounting sense of discomfort or anxiety relieved only by the performance of a tic. Premonitory urges are similar to the sensation preceding a sneeze or an itch. Premonitory urges cause many TS patients to suffer from an endless cycle of rising tension and tic performance because the relief provided by tic performance is ephemeral. Thus, soon after tic performance the tension of the premonitory urge again rises to a crescendo. A majority of patients also report a fleeting sense of relief after a bout of tics has occurred. Most are able to suppress their tics for short intervals of time.
With increasing awareness of premonitory urges, TS patients begin to exhibit a variable degree of voluntary control over tic performance. 92% of TS subjects in one study reported that the tics they exhibited were either partially or totally voluntary. However, this voluntary control should be likened to that governing eye blinking. Eye blinking and tics can both be inhibited voluntarily, but only for a limited period of time and only with mounting discomfort. Thus, some adult TS patients are able to demonstrate nearly complete control over when their tics will occur. However, when complete or near complete control of tics is present, resistance to the mounting tension of premonitory urges can produce mental and physical exhaustion even more distracting than the tics themselves.
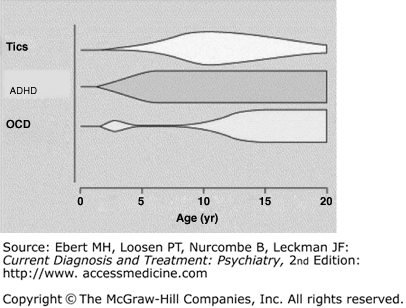
The tics, which are the most prominent feature of TS, may be neither the first nor the most impairing psychological disturbance TS patients endure. Children with TS have higher rates of OCD, ADHD, and disinhibited speech and behavior compared to the general population. In one study, 65% of TS patients in late adolescence regarded their behavioral problems (including ADHD and OCD) and learning difficulties to have had an equal or greater impact on their life function than the tics themselves did. In the natural course of comorbid psychiatric illness in TS, ADHD symptom, typically precede the onset of tic symptoms by a couple of years, whereas OCD symptoms typically present around the age of 12–13 after tics have reached their peak severity. Approximately 50% of children with TS experience comorbid ADHD, and an even greater proportion of children with comorbid disorders reach clinical attention. Roughly one-third to one-half of TS patients will experience clinically significant OCD symptoms during the course of their lifetime. Figure 44–1 depicts the clinical course of comorbid ADHD and OCD symptoms in children with tic disorders.
Direct observational methods (e.g., videotaped tic–counting) are the most objective measures of tic severity. However, the frequency of tics varies dramatically according to setting and activity. In addition, many individuals with TS can suppress their symptoms for brief periods of time. Thus, clinical rating scales are the preferred method for assessing initial tic severity and measuring change in tic severity. The Yale Global Tic Severity Scale is a clinician-rated, semi-structured scale that begins with a systematic inventory of tic symptoms rated as present or absent over the past week. Current motor and phonic tics are then rated separately according to number, frequency, intensity, complexity, and interference, in accordance with 6-point ordinal scales (0 = absent; 1 through 5 for severity). Three scores are yielded: Total Motor, Total Phonic and Total Tic. Self-report inventories such as the Yale Child Study Center TS Obsessive–Compulsive Disorder Symptom Questionnaire can be completed by the family prior to their initial consultation. They are valuable ancillary tools in order to gain a long−term perspective of the child’s developmental course and the natural history of the tic disorder. The Yale Global Tic Severity Scale and Yale Child Study Center TS Obsessive–Compulsive Disorder Symptom Questionnaire are freely available.
At present, no laboratory testing or neuroimaging is useful in diagnosing or treating tic disorders. However, strong evidence from neuroimaging, neuropathological and neurochemical studies implicates abnormalities in the basal ganglia and CSTC circuits in the pathogenesis of TS. On volumetric MRI, individuals with TS have smaller caudate volume than healthy controls. Reduced caudate volume in children with TS has been associated with increased tic and OCD severity in adulthood. Functional neuroimaging has revealed increased activation of the frontal cortex and caudate during willful tic suppression. This increased activation of frontal cortex and caudate is in turn correlated with decreased activity of the globus pallidus, putamen, and thalamus. Positron emission tomography studies in TS have demonstrated increased striatal dopamine receptor and transporter densities and increased amphetamine induced dopamine release in the putamen.
Tic disorders must be distinguished from movement disorders caused by general medical conditions (e.g., Huntington’s disease, stroke, Lesch-Nyhan syndrome, Wilson’s disease, Sydenham’s chorea, multiple sclerosis, postviral encephalitis, head injury) or the direct effects of a drug (e.g. akathisia and tardive dyskinesia associated with neuroleptic medication). Table 44–1 defines the major types of movements that commonly need to be distinguished from tics. Medical and family history, movement morphology, rhythm, and modifying influences are usually sufficient for making the diagnosis. Onset of movements prior to age 18, a waxing-and-waning course of symptoms and the changing of the specific tic movements over time are characteristic of TS.
Movement | Description | Common Causes |
|---|---|---|
Tics | Abrupt, stereotyped coordinated movements or vocalizations that often mimic aspects of regular behavior. Premonitory urges are common. Exacerbated by stress and relieved by distraction. | TS, Chronic Tic Disorder, Transient Tic Disorder |
Stereotypies | Repetitive, purposeless, and apparently voluntary movements | Autism, Pervasive Developmental Disorder, Mental Retardation, Stereotyped Movement Disorder |
Chorea | Simple, random, irregular, and nonstereotyped movements. Have no premonitory component and increase when the person is distracted. Often flows from one body part to another. | Normal in children less than 8 months of age. Cerebral Palsy, Sydenham’s Chorea, Hereditary choreas, kernicteris, Lesch-Nyhan syndrome, hypoxia or stroke. |
Dyskinesia | Slow, protracted twisting movements interspersed with prolonged states of muscular tension | Drug-induced, idiopathic torsion dystonia, anoxia or stroke, Wilson’s disease, Huntington’s Disease and Parkinson’s Disease |
Athetoid | Slow, irregular, writhing movements. Usually involving fingers and toes but occasionally the neck. A “slow chorea.” | See chorea |
Myoclonia | Brief, simple, shock-like muscle contractions that may affect individualized muscles of muscle groups. |