Fig. 9.1
Simplified procedures of harvesting, processing, culturing, and characterization of adipose-derived stem cells. After being removed from the human body through procedures such as liposuction and lipectomy, the adipose tissue is minced to small pieces of size less than 1 mm3 to maximize the efficiency of enzymatic digestion for freeing the cells from connective tissue. After cell-harvesting through filtration and centrifugation, the cells are purified with the number of cells expanded through culturing. The cultured cells subsequently undergo flow cytometric analysis for the identification of surface markers characteristic of mesenchymal stem cells. (Note the typical spindle-shaped morphology of mesenchymal stem cells at right lower corner)
9.2.4 Automated Devices for Adipose-Derived Stem Cell Isolation
Compared with other tissues from which stem cells are isolated, adipose tissue has been shown to have at least two log greater concentrations of available stem and progenitor cells. This knowledge enables the direct utilization of these useful cellular elements without prior ex vivo expansion (Hicok and Hedrick 2011). Indeed, the Celution system, which is a closed, commercially available automated platform for adipose tissue processing for the isolation of adipose-derived stem and progenitor cells, has been described in 2011 (Hicok and Hedrick 2011). The system has been reported to take only 2.5 h for processing and successfully applied clinically (Marino et al. 2013). The “stromal vascular fraction” (SVF) thus obtained comprises both live and dead cells. Therefore, one noteworthy concern is that the cell debris may contribute to subsequent inflammatory responses that would potentially alter cell differentiation (Ye and Gimble 2011). Accordingly, several approaches have been proposed for retrieving the viable cells from SVF, including fluorescence-activated cell sorting (FACS), magnetic activated cell sorting (MACS), and dielectrophoresis (Wu and Morrow 2012). The former two involve the use of antibodies, while the latter retrieves life cells based on the presence of charge on their surface.
9.3 ADSC as a Therapeutic Option Against Stroke: Principles and Mechanisms
9.3.1 Therapeutic Actions of ADSC Implicated in Pathophysiological Changes of Stroke
The therapeutic role of ADSC against stroke could best be understood by reviewing the essential pathological changes and the physiological recovery mechanisms involved. Ischemia-induced inflammatory responses in stroke involve not just the neurons, but also other components of the neurovascular unit (del Zoppo 2009). This finding underscores the importance of immunomodulation in the management of stroke instead of merely restoring tissue perfusion (Iadecola and Anrather 2011). In addition, investigation of brain recovery from ischemic stroke has revealed the plasticity of the repairing process that involves axonal outgrowth and myelination (Ueno et al. 2012). Moreover, beside necrosis, apoptosis initiated after the stroke attack also results in irreversible loss of cellular elements in the central nervous system (Ouyang and Giffard 2013). Furthermore, although resuming patency of the obstructed vessel through fibrinolysis or angioplasty theoretically salvages the region at risk of ischemic infarction, the resulting ischemia-reperfusion injury actually triggers a cascade of inflammatory events (Iadecola and Anrather 2011; Liu et al. 2014). The major contributor to injuries following reperfusion is the reactive oxygen species (ROS) generated both from inflammatory cells and damaged mitochondria (Manzanero et al. 2013).
Pathologically, similar to the microscopic changes observed in animal models of stroke, evidence of reactive gliosis has been reported in human subjects after ischemic stroke including increased numbers of glial fibrillary acidic protein (GFAP)-positive reactive astrocytes and ED1-positive activated microglia as well as enhanced expression of chondroitin sulphate proteoglycans (CSPG) in the cortical penumbra regions (Huang et al. 2014). Hence, stroke involves a series of pathological changes that require a number of corresponding measures for the subsequent repairing. This is reflected in the results of a previous study that demonstrated the activation of hundreds of genes responsible for not only tissue repair, but also nervous system development and cell proliferation both in the penumbra and core of infarct as early as 24 h after ischemic stroke in rats (Ramos-Cejudo et al. 2012), highlighting the complexity of the repairing process. Since it is proposed that stem cells , which are known to participate in physiological tissue repair in various organs, may have a significant role to play in the recovery process after stroke (Gutierrez-Fernandez et al. 2012), numerous previous studies have been conducted to investigate the therapeutic potential of ADSC using the known recovery mechanisms of stroke as referring parameters.
9.3.2 Observed Therapeutic Effects of ADSC Against Stroke
To date, most results of the therapeutic use of ADSC against stroke came from animal studies for which middle cerebral artery occlusion (MCAO) is the commonly used model. The parameters for assessment were based on the established pathological changes after stroke at molecular, cellular, and functional levels. For instance, the findings of increased levels of chemokine receptor type 4 (CXCR4), stromal cell-derived factor 1 (SDF-1), IL-8/Gro, Doublecortin (DCX) (i.e., marker of migrating neuroblasts), von Willebran factor (vWF), and endothelial cell markers as well as enhanced microvessel proliferation after ADSC treatment in a rat ischemic stroke model in one study (Leu et al. 2010), together with consistent observation of augmented expressions of basic fibroblast growth factor (bFGF) and VEGF with enhanced angiogenesis in the brain in another animal investigation (Wang et al. 2008), highlight the roles of ADSC in nerve repair and revascularization in the ischemic brain. Reinforcing evidence was provided by another study that demonstrated elevated levels of VEGF, synaptophysin (SYP), oligodendrocyte (Olig-2) and neurofilament (NF) in rats after ADSC treatment compared to those in untreated animals 14 days after MCAO (Gutierrez-Fernandez et al. 2013b). The reduction in expression of GFAP in the previous studies also signifies an amelioration of reactive gliosis after ADSC treatment (Leu et al. 2010; Gutierrez-Fernandez et al. 2013b; Jiang et al. 2014). Besides, the suppressed mRNA expressions of Bax and caspase 3 as well as the increased expression of Bcl-2 in animals with stroke after ADSC treatment compared to those in the untreated group suggest an anti-apoptotic function of ADSC (Leu et al. 2010; Jiang et al. 2014). On the other hand, intravenous infusion of human ADSC has also been reported to attenuate neurological deficits (Kim et al. 2007; Yang et al. 2012), brain edema, atrophy, glial proliferation , inflammation , and apoptosis (Kim et al. 2007) in a rat model of hemorrhagic stroke.
However, the effect of ADSC treatment on infarct volume after experimental ischemic stroke is equivocal. Although one study demonstrated a significant reduction (Leu et al. 2010), other studies demonstrated no notable change in infarct volume (Gutierrez-Fernandez et al. 2013b; Jiang et al. 2014) despite the same number of cells being administered each time (2 × 106) and the unanimous findings of significantly improved neurological function, reduced cell death, and enhanced cellular proliferation in all studies (Leu et al. 2010; Gutierrez-Fernandez et al. 2013b; Jiang et al. 2014). The discrepancies in infarct volume among the studies may partly be explained by the differences in the choice of ligation procedure (i.e. permanent (Gutierrez-Fernandez et al. 2013b) vs. transient (Leu et al. 2010; Jiang et al. 2014)), the timing and frequency of ADSC administration (once at 30 min after stroke (Gutierrez-Fernandez et al. 2013b) vs. 3 times at 0, 12 and 24 h after stroke (Leu et al. 2010) vs. once at 3 days after stroke induction (Jiang et al. 2014)), the time of sacrificing animals for histological analysis after induction (14 days (Gutierrez-Fernandez et al. 2013b) vs. 21 days (Leu et al. 2010) vs. 28 days (Jiang et al. 2014)), and the route of ADSC administration (systemic intravenous (Leu et al. 2010; Gutierrez-Fernandez et al., 2013b) vs. intra-carotid arterial (Jiang et al. 2014)). Therefore, although the timing of sacrificing animals and the route of cell injection do not seem to be the significant causes of discrepancies in the size of infarct, it appears that an early timing and increased frequency of ADSC administration (Leu et al. 2010) offered significant benefit in the reduction of infarct volume in a rodent experimental setting of ischemic stroke. Accordingly, a meta-analysis demonstrated that the efficacy in structural restoration drops by 1.5 % for each day delay in treatment and that a significant dose-response relationship exists between the number of stem cells administered and the improvement of structural outcome after ischemic stroke (Lees et al. 2012). Another interesting comparison based on animal experimentation between the therapeutic effects of autologous and allogeneic cells on structural and functional outcomes after ischemic stroke revealed that the former is more effective in preserving structural integrity, while the latter is more beneficial for functional outcome (Lees et al. 2012).
9.3.3 Mechanisms Underlying Therapeutic Actions of ADSC from Experimental Studies
Taken together, experimental investigations, both in vitro and in vivo, have provided significant insight into some of the mechanisms involved in repair of the nervous system after stroke. The mechanisms underlying the above-mentioned therapeutic benefits of ADSC against stroke can be summarized into (i) paracrine effects, (ii) transdifferentiation, and (iii) immunomodulation (Fig. 9.2) .
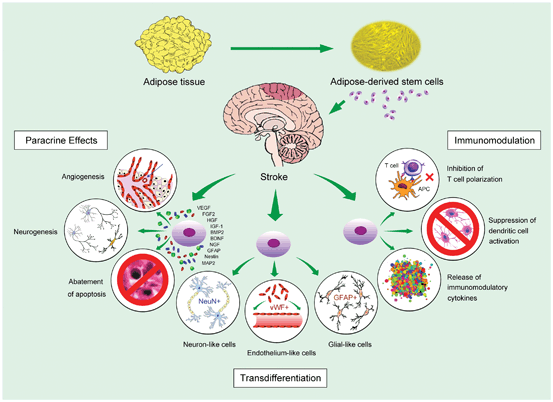
Fig. 9.2
Summary of reported mechanisms underlying adipose-derived stem cell treatment for stroke. The three major mechanisms by which adipose-derived stem cells exert therapeutic functions include paracrine effects , transdifferentiation, and immunomodulation . The paracrine effects stem from the release of a variety of trophic factors from stem cells that elicit a number of biological responses such as angiogenesis, neurogenesis, and abatement of apoptosis. Transdifferentiation of stem cells involves the transformation of implanted stem cells into specific cellular elements with distinct functions and cell markers (e.g., neuron-like, endothelium-like, or glial-like cells). Immunomodulation includes stem cell-mediated modification of the immunological system, such as inhibition of T cell polarization for alleviating immune responses, suppression of transformation of monocytes to antigen-presenting immunogenic cells (e.g., dendritic cells) for inducing tolerance, and the release of various immuomodulatory cytokines for suppressing inflammatory reactions. APC: Antigen-presenting cell; VEGF: Vascular endothelial growth factor; FGF2: Fibroblast growth factor 2; HGF: Hepatocyte growth factor; IGF-1: Insulin-like growth factor-1; BMP2: Bone morphogenetic protein 2; BDNF: Brain-derived neurotrophic factor; NGF: Nerve growth factor; GFAP: Glial fibrillary acidic protein; MAP2: Microtubule-associated protein 2; NeuN: Neuronal nuclei; vWF: von Willebran factor
9.3.3.1 Paracrine Effects
Although several previous experimental studies have demonstrated the presence of ADSC in the brain up to several weeks after being administered , the scarcity of stem cells in brain tissue could not account for the observed therapeutic outcomes (Leu et al. 2010; Jiang et al. 2014). The finding of stem cells not yet embedded into the brain tissue (Gutierrez-Fernandez et al. 2011; Ikegame et al. 2011) also precludes the possibility of their direct participation as fully functional neurons, implying their help through other mechanisms in the recovery process (Gutierrez-Fernandez et al. 2011). In concert with that finding, ADSC has been reported to produce a number of trophic factors including VEGF, angiopoietin-1, and HGF (Ikegame et al. 2011), insulin-like growth factor-1 (IGF-1) (Wei et al. 2009), TGF-β1 (Melief et al. 2013b), bone morphogenetic protein 2 (BMP2) and fibroblast growth factor 2 (FGF2) (Moriyama et al. 2012) as well as nervous system-related molecules including nerve growth factor (Banas et al. 2008; Ikegame et al. 2011), brain-derived neurotrophic factor (BDNF) (Iadecola and Anrather 2011; Liu et al. 2014), GFAP, nestin, and microtubule-associated protein 2 (MAP2) (Yang et al. 2011). Therefore, based on the actions of these trophic factors, it is rational to attribute the observed enhancement of angiogenesis and neurogenesis as well as the abatement of apoptosis to the paracrine effects of the administered ADSC (Leu et al. 2010; Gutierrez-Fernandez et al. 2012) .
Consistently, another intriguing finding is the discovery of therapeutic effects against stroke using cell-free ADSC culture medium (Cho et al. 2012; Egashira et al. 2012). One study applying human adipose-derived stem cell-conditioned medium to the lateral ventricle of a rat model of ischemic stroke 8 h after MCAO continuously for 7 days demonstrated not only a reduction of infarction volume and preservation of motor function, but also enhanced endothelial cell proliferation , reduced neural cell apoptosis, and suppressed astrogliosis in the penumbra regions (Cho et al. 2012). Another similar study using intracerebroventricular administration of concentrated murine adipose-derived stem cell-conditioned medium in a murine model of MCAO-induced ischemic stroke shed some light on the importance of the timing of treatment (Egashira et al. 2012). The result of that study showed that, while administration of conditioned medium prior to MACO exhibited a dose-dependent reduction in infarction volume of the brain and administration 5 min after MACO was still effective, the therapeutic effect vanished if conditioned medium was administered 2 h after MCAO (Egashira et al. 2012). By contrast, the former study reported effectiveness up to 8 h after MCAO before starting conditioned medium treatment (Cho et al. 2012). Other than the possible variations arising from the differences in the source of conditioned medium and the animal model used, the discrepancy in therapeutic effects between the two studies appears to be due to the way of conditioned medium administration. While the former adopted the approach of continuous intracerebroventricular infusion (Cho et al. 2012), the latter used single intracerebroventricular injection (Egashira et al. 2012) . Again, consistent with the results of previous experimental studies using ADSC transplantation for ischemic stroke (Leu et al. 2010; Gutierrez-Fernandez et al. 2012), it appears that early timing and repeated (if not continuous) treatment are of therapeutic advantage for both ADSC transplantation and conditioned medium therapy. In vitro, murine ADSC-derived conditioned medium has also been demonstrated to reduce glutamate-induced excitotoxicity in human neuroblastoma cells (Egashira et al. 2012).
9.3.3.2 Transdifferentiation
The role of direct cell participation regarding the use of ADSC for the treatment of ischemic stroke remains controversial. Previous studies using bone marrow-derived mesenchymal stem cells demonstrated that physical presence of the infused stem cells depends on the route of administration. Implantation of stem cells in the injured brain was evident when the cells were given through the carotid artery (Gutierrez-Fernandez et al. 2011; Jiang et al. 2014) but not through the intravenous route (Gutierrez-Fernandez et al. 2011) . Neurological deficits, however, were improved regardless of the presence of implanted stem cells in the brain (Gutierrez-Fernandez et al. 2011; Jiang et al. 2014), raising the question regarding the therapeutic significance of stem cell implantation in stroke. Indeed, it has been shown that only a small fraction (around 0.02 %) of intravenously administered bone marrow-derived hematopoietic stem cells migrate to the ischemic brain, and most of the transplanted cells express microglial but not neural protein markers (Schwarting et al. 2008). For ADSC, while a study failed to identify evidence of migration or implantation of cells into the damaged brain after their intravenous injection in an animal model of stroke despite significant functional recovery (Gutierrez-Fernandez et al. 2013b), other experimental studies (Kim et al. 2007; Leu et al. 2010; Yang et al. 2012) have demonstrated presence of the transplanted ADSC several weeks after intravenous administration with the expression of von Willebran factor, a marker of endothelial cell (Kim et al. 2007; Leu et al. 2010) . Another study using ADSC to treat a rat model of hemorrhagic stroke through right lateral cerebral ventricular injection demonstrated the differentiation of the infused ADSC into neuron-like (NeuN+) and glial-like cells (GFAP+) in region surrounding the hematoma (Chen et al. 2012). Despite the relatively small number of ADSC to explain the overall functional recovery in the reported studies, their presence signifies “transdifferentitation” as a possible mechanism underlying the positive therapeutic impact (Gutierrez-Fernandez et al. 2013a). Indeed, the capacity of neural differentiation for ADSC has been extensively investigated (Cardozo et al. 2010; Kompisch et al. 2010; Liao et al. 2010; Qian et al. 2010; Abdanipour et al. 2011; Yu et al. 2011; Ahmadi et al. 2012). It has also been reported that, compared with bone marrow-derived mesenchymal stem cells , ADSC have superior neurogenic potential (Kang et al. 2004) . Consistently, previous studies using ADSC after induced neural differentiation for treating experimental ischemic stroke were also found to be effective in improving functional recovery (Kang et al. 2003b; Yang et al. 2011). On the other hand, another finding of interest is the requirement for direct physical contact between human ADSC and murine neural stem cells in vitro for induction of neuronal differentiation of the latter, further emphasizing the existence of a mechanism that involves cell-cell interaction other than that of transdifferentiation and paracrine effects in promoting neurogenesis (Kang et al. 2003a) .
9.3.3.3 Immunomodulation
Taking into account the immunological nature of stroke-elicited damage and the subsequent repairing process (Iadecola and Anrather 2011) , it is not surprising to find that ADSC exert their therapeutic actions at least partly through immunomodulation. Indeed, ADSC have been reported to produce a variety of immunomodulatory cytokines, including IL-1R, IL-6, IL-8, IL-18, toll-like receptor (TLR)-4, TGF-β1, plasminogen activator inhibitor-1 (PAI-1), G-CSF, GM-CSF, and monocyte chemotactic protein 1 (Banas et al. 2008; Leu et al. 2010; Ikegame et al. 2011; Melief et al. 2013b). Moreover, ADSC have been shown to suppress the differentiation of monocytes towards antigen-presenting immunogenic cells and promote differentiation towards an anti-inflammatory IL-10-producing cell type through the production of IL-6 (Melief et al. 2013a). Consistently, coculturing ADSC with allogeneic dendritic cells revealed that ADSC could negatively modulate immunity and induce immune tolerance through downregulating costimulatory molecules (i.e., CD80, CD83, CD86, and secretion of IL-12 and tumor necrosis factor (TNF)-alpha), while induce dendritic cell tolerance through upregulating indoleamine-2,3-dioxygenase (IDO). Cocultured dendritic cells were also found to inhibit CD4+ T cell activation and naive T cells toward Th1 helper cell polarization (Peng et al. 2012). Again, another credit given to ADSC as compared with bone marrow-derived mesenchymal stem cells in the aspect of immunomodulation in stroke treatment is the finding of a higher immunomodulatory capacity in the former than that in the latter (Melief et al. 2013b) .
9.4 Clinical Use of ADSC Against Stroke: Present Status, Perspectives, and Limitations
9.4.1 Clinical Application of ADSC: Probabilities and Possibilities
Given the promising experimental outcomes of applying stem cells to the treatment of stroke and the in-depth understanding of the underlying mechanisms, a number of clinical trials are either reported or still on-going in recent years despite the majority of them are small, nonrandomized, and uncontrolled. The cells administered included bone marrow mononuclear cells (Correa et al. 2005; Li et al. 2013b), bone marrow-derived mesenchymal stem cells, (Bang et al. 2005; Suarez-Monteagudo et al. 2009; Lee et al. 2010; Bringas et al. 2011; Honmou et al. 2011), human teratocarcinoma-derived neurons (Kondziolka et al. 2000), peripheral blood hematopoietic progenitor/stem cells (Chen et al. 2014a), umbilical cord-derived mesenchymal stem cells (Han et al. 2011; Jiang et al. 2013), as well as human (Rabinovich et al. 2005) and porcine fetal cells (Savitz et al. 2005). Except for premature termination of the study adopting porcine fetal cells because of overt complications (Savitz et al. 2005), the results of other published trials support the safety and effectiveness of stem cell/progenitor cells as a therapeutic tool in the clinical setting of ischemic and hemorrhagic stroke as reflected in the overall significantly improved neurological functions of the treated patients up to 5 years of follow-up (Lee et al. 2010). On the other hand, results on the use of ADSC in clinical trial have not been reported. To date, there is only one study still recruiting patients to explore the safety and effectiveness of applying autologous ADSC in patients after stroke on the National Institutes of Health clinical trial registry database (www.clinicaltrials.gov). Therefore, albeit optimistic, the exact therapeutic impact of ADSC on disease progression and functional recovery in the clinical setting of stroke remains to be elucidated for the years to come.
9.4.2 ADSC Against Stroke: Concerns and Speculations
Despite the promising outcomes of applying ADSC to the treatment of stroke in experimental settings, there have been serious concerns about possible tumorigenesis in the clinical scenario because of the multilineage differentiation potential of ADSC (Lee et al. 2012). A study investigating the fate of human ADSC from different human donors after being subcutaneously injected into immunodeficient SCID mice showed that the cells survived for at least 17 months with subsequent differentiation into fibroblasts of the subdermic connective tissue and into mature adipocytes of fat tissue, exclusively at the site of injection without evidence of migration or fusion with host cells (Lopez-Iglesias et al. 2011), underscoring the safety of ADSC transplantation. Moreover, the use of terminally differentiated ADSC may be a possible option for minimizing the risk especially when the protocols for in vitro transdifferentiation of ADSC into neuronal lineage have been well-documented (Cardozo et al. 2010; Kompisch et al. 2010; Liao et al. 2010; Qian et al. 2010; Abdanipour et al. 2011; Yu et al. 2011; Ahmadi et al. 2012). Indeed, the use of induced ADSC has been endorsed as a promising therapeutic option in stroke treatment (Yang et al. 2011; Shen et al. 2013). Furthermore, it has been shown that iPSC can be generated from human ADSC without transducing c-myc so that the proliferative and differentiation capacity of ADSC can be enhanced without increasing the risk of oncogenesis (Aoki et al. 2010).
On the other hand, taken into account the therapeutic advantage of early stem cell administration at the acute stage of stroke, the use of automated devices for adipose-derived stem cell isolation for direct injection without ex vivo expansion and purification may be a feasible option for daily clinical practice because of the high concentration of useful stem and progenitor cells from adipose tissue compared with other sources (Hicok and Hedrick 2011). At the other end of the spectrum, the use of gene-transfer techniques for producing stem cells over-expressing different neurotrophic factors, such as BDNF, glial derived neurotrophic factor (GDNF), or neurotrophin-3 (NT-3), has been reported to be effective options in the treatment of ischemic stroke in animal models (Chen et al. 2013). Finally, considering the wide therapeutic applicability and easy harvesting of ADSC, the establishment of autologous or allogeneic cell banks for ADSC storage to facilitate urgent or scheduled use is no longer a far-fetched idea (West et al. 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








