Fig. 23.1
Photomicrograph showing the distribution of PKH26-labeled transplanted MSC counterstained with DAPI staining for nuclei at 1 and 5 weeks after spinal cord injury. Transplanted PKH26-induced fluorescent MSCs survived and appeared only around the injured site at 1 week after spinal cord injury (c), whereas a number of PKH-positive cells extended out of the injured lesion at 5 weeks after spinal cord injury (a, b). (b) High-power photomicrograph of the box area of (a). Scale bar = 1 mm (a, c), 50 μm (b) (Reprinted, with permission, from [14])
Immunostaining of spinal cord sections for NeuN, RIP, GFAP, and OX-42 was performed at 5 weeks after transplantation of MSC. There was no obvious colocalization of NeuN, RIP, GFAP, or OX-42 with PKH26-identified MSC. These results indicated that although the MSCs were distributed in areas rich in glial and neuronal processes, there was no evidence that the MSCs themselves had transdifferentiated into glial or neuronal cells.
23.1.2 Effects of MSC Transplantation on Phenotype of Infiltrated Macrophages After Spinal Cord Injury
Higher numbers of classically activated macrophages located mainly in the gray matter around the site of injury, co-expressing iNOS, CD16/32, and OX-42, were found in the control group (82.5 ± 5.8 % and 78.7 ± 7.1 % of OX42-positive cells, respectively) compared with the MSC-transplanted group (27.4 ± 5.1 % and 19.6 ± 4.2 % of the OX42-positive cells, respectively). On the other hand, consistently higher numbers of alternatively activated macrophages, co-expressing arginase-1/CD206 and OX-42, were found in the MSC-transplanted group (38.9 ± 8.1 % and 32.1 ± 6.6 % of the OX42-positive cells, respectively), with a more localized distribution at the site of injury. On the other hand, only a few arginase-1/CD206/OX42-positive cells were seen in the control group (1.2 ± 0.1 % and 0.8 ± 0.05 % of the OX42-positive cells, respectively) (Fig. 23.2).
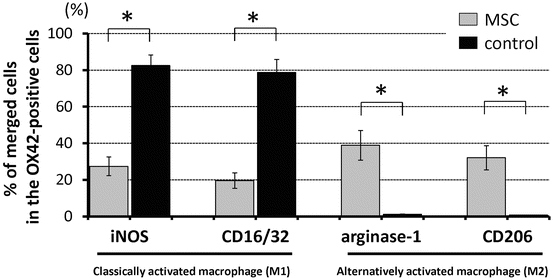

Fig. 23.2
The graph showing differences in the expression of iNOS and CD16/32 for classically activated macrophages (M1 phenotype) and arginase-1 and CD206 for alternatively activated macrophages (M2 phenotype) colocalized OX-42 after MSC transplantation in the injured spinal cord at 1 week after spinal cord injury. Percentage of merged cells of OX42-positive cells in the area 0–1 mm caudal and rostral to the epicenter. Data are mean ± SD. *p < 0.05 (Reprinted, in part, with permission, from [14])
The profile of the identified macrophages (CD45positive/CD11bhigh/GR-1negative cells) was also analyzed quantitatively using flow cytometry. The proportions of CD45positive/CD11bhigh/GR-1negative cells (macrophages) within the injured spinal cord were higher in both the MSC (24.5 ± 1.9 %) and control group (30.4 ± 2.1 %) compared with the sham (laminectomy only) group (4.5 ± 1.3 %) at 1 week after SCI. In the control group, at 1 week after SCI, 93.4 ± 5.6 % of these cells were iNOSpositive classically activated macrophages (M1 phenotype; 70,984 ± 4,256 cells) whereas no arginase-1positive alternatively activated macrophages (M2 phenotype) were present. In comparison, in the MSC-transplanted group, only 11.7 ± 1.3 % of these cells were iNOSpositive (7,166 ± 796 cells) and 32.2 ± 1.9 % were arginase-1positive (19,723 ± 1,164 cells) (Fig. 23.3a, b). The difference in the proportions of iNOSpositive and arginase-1positive cells between the MSC-transplanted and control group indicated that MSC transplantation seems to reduce the classically activated macrophages (M1 phenotype) and shift the macrophage phenotype to the alternatively activated macrophages (M2 phenotype).
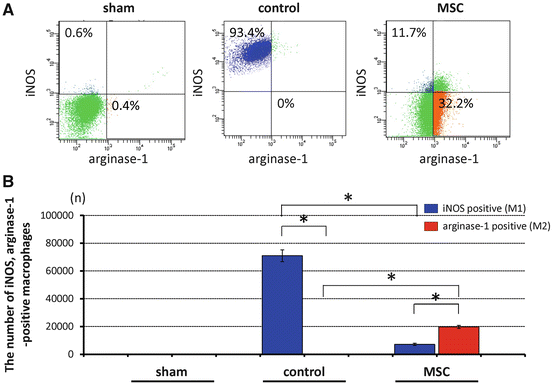

Fig. 23.3
Representative flow cytometry data at 1 week after injury. The number of iNOSpositive or arginase-1positive cells within the CD45positive/CD11bhigh/GR-1negative (macrophages) populations is different between MSC-transplanted and control groups (a). Number of iNOS- and arginase-1-positive cells in 250,000 analyzed cells. MSC transplantation reduced the proportion of iNOSpositive cells (blue, M1 phenotype) and shifted the macrophage phenotype to arginase-1positive cells (red, M2 phenotype) at 1 week after spinal cord injury (b). Data are mean ± SD. *p < 0.05 (Reprinted, with permission, from [14])
23.1.3 Changes in Cytokine Expression After MSC Transplantation by Immunoblot Analysis and ELISA
Western blotting and ELISA were performed to evaluate the effects of MSC transplantation on TNF-α, IL-6, IL-4, and IL-13 protein levels in the region of the SCI at 1 week after injury. In the MSC-transplanted group, the intensities of the bands for TNF-α and IL-6 were attenuated, whereas those of IL-4 and IL-13 were increased compared with the control group. The amounts of TNF-α and IL-6 proteins were significantly lower (IL-6 dominant) while those of IL-4 and IL-13 were significantly higher (IL-13 dominant) in the MSC-transplanted group compared with the control group.
23.1.4 Histological Evaluation of Injured Spinal Cord After MSC Transplantation
In order to examine the beneficial effects of MSC transplantation on spinal cord repair, the severity of trauma at the injury epicenter site was evaluated histopathologically at 5 weeks after SCI. Hematoxylin and eosin staining in the MSC-transplanted group showed a significant decrease in the total cavity areas at epicenter and at 4 mm rostral and caudal to the epicenter compared with the control group (Fig. 23.4a). On LFB-stained samples, images of the MSC-transplanted group demonstrated significantly smaller areas of cystic cavity formation and enhanced staining in both gray and white matters compared with that of the control group. Quantitative analysis of the myelinated areas revealed significant differences between the two groups at the injury epicenter and at 4 mm rostral and caudal to the epicenter (Fig. 23.4b). To determine the effects of MSC transplantation on axonal sparing, we examined sagittal sections of the spinal cords following immunostaining with anti-GAP-43 and anti-RT97 antibodies at 5 weeks after SCI. In the MSC-transplanted group, GAP-43-positive or RT97-positive fibers, embedded within PKH26-labeled MSC, were significantly increased and their neurites were also significantly longer compared with the control group (Fig. 23.4c).
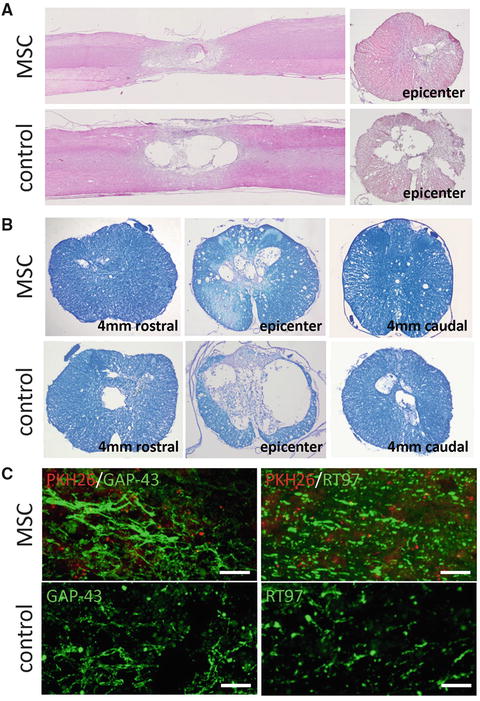

Fig. 23.4
Hematoxylin and eosin staining of midsagittal and axial sections of the lesion epicenter showed a remarkably smaller area of damage and cavity formation in the MSC-transplanted group. The total area of cavitation in the axial sections was significantly different between the two groups (a). The axial sections stained with Luxol fast blue (LFB) contained remarkably small area of demyelination in the MSC-transplanted group compared with the control group. Quantification of LFB-positive myelinated area showed a significant difference between the two groups at all examined sites (b). Representative images showing greater abundance of GAP-43-positive fibers and RT97-positive fibers in the MSC group compared with that in the control group (5 weeks after injury). Quantification of GAP-43- and RT97-positive fibers areas showed a significant difference between the two groups at all examined sites (c). Scale bar = 100 μm (Reprinted, in part, with permission, from [14])
23.1.5 Changes in Locomotor BBB Score
The degree of motor disturbance in the hind limbs was assessed between day 3 and 5 weeks after SCI. Significant motor disturbance in the hind limbs was noted in rats of the control group, though some degree of recovery was evident subsequently, which reached a functional plateau (BBB score 9.2 ± 2.1) [13] at 5 weeks after SCI. In contrast, the MSC-transplanted group showed a markedly better functional recovery such that the BBB locomotor score was significantly increased compared to the control group from 1 week after SCI (Fig. 23.5).
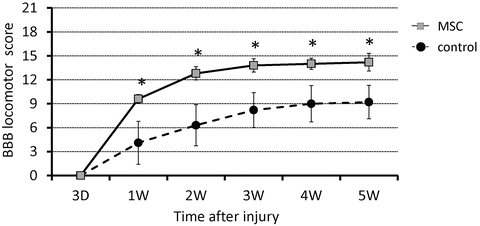

Fig. 23.5
Serial changes in locomotor BBB score after SCI. A significant improvement in hindlimb motor function was observed in the MSC-transplanted group compared with the control group from 1 week and thereafter after the injury. *p < 0.05 (Reprinted, with permission, from [14])
23.2 Discussion
The aim of this research was to study the beneficial effects of MSC transplantation in the injured spinal cord, specifically with regard to the effects of MSC on macrophage phenotype and function. The major findings of our research were: (1) MSC-transplanted into the site of spinal cord injury migrated into the adjacent nervous tissue to areas rich in glial and neuronal processes, but did not differentiate into glial or neuronal elements; (2) MSC transplantation favored the development of a population of alternatively activated macrophages (M2 phenotype) while preventing the development of a population of classically activated macrophages (M1 phenotype); (3) MSC transplantation was associated with a decrease in TNF-α and IL-6 and an increase in IL-4 and IL-13; (4) MSC transplantation reduced the size of injury site and resulted in less scar tissue formation and increased myelin sparing; and (5) these morphological findings correlated with increased axonal growth and improved locomotor function in the MSC-transplanted group compared with the control group [14].
Several studies reported that MSC can differentiate in vitro into neurons, astrocytes, myocytes, and Schwann cells. In our study, transplanted MSC migrated to the neighborhood of the injured spinal cord, but did not differentiate into glial or neuronal elements. Current thinking is that the potential beneficial effects of MSC in SCI are related to neuronal or glial differentiation of MSC, but rather from their secretion of growth factors and/or cytokines [15], which can provide neuroprotection [16], induction of axonal sprouting [17], neovascularization [18], and immunomodulation [19, 20]. MSC may also promote axonal regeneration or encourage functional plasticity by establishing an environment that supports axonal growth. MSC synthesize a number of neurotrophic cytokines that stimulate nerve growth, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) [8, 21]. Furthermore, a recent study examined the response of MSC to environmental stimuli in the injured spinal cord tissue and found increased synthesis by these cells of various cytokines, including IL-6, IL-7, and VEGF [22]. Because MSC can alter their gene expression profile in response to the surrounding environment [23, 24], we consider that transplanted MSC do not differentiate into neural cells at least in the injured spinal cord environment, but instead they bring about CNS functional recovery by modifying the SCI environment to directly affect the endogenous cells present in that territory.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








