Fig. 13.1
Detrimental effects of secondary spinal cord injury events at the cellular and molecular level. Neurons are shown in green; glia in red. Injury to the spinal cord leads to (1) apoptosis and cell death (neurons and glial cells) with accumulation of cell debris and the generation of a toxic environment due to high extracellular glutamate concentrations and the presence of free radicals; (2) formation of a glial scar which creates a physical barrier consisting of activated astrocytes. The resultant effect is that damaged axons are unable to regenerate and communicate due to presence of inhibitory molecules, loss of myelin and the physical barrier
Current therapies for spinal cord injury do not lead to significant neural regeneration and functional recovery. Most of these therapies have aimed to minimize the post-traumatic cell damage but fail to achieve the re-establishment of neuronal connections. Drug therapy is generally applied immediately following trauma to treat inflammation and initial degeneration (reviewed by Stahel et al. 2012; Batzofin et al. 2013; Hurlbert et al. 2013). This treatment is often followed by long term therapies aimed at promoting axonal growth and neutralising the toxic environment at the injury site. A major factor hampering axonal regeneration following spinal cord injury is the down-regulation of endogenous neurotrophins and one method that has shown promise is the injection of neurotrophins at the site of injury to replace the lost endogenous neurotrophins (Hulsebosch 2002). Peripheral glial cells can produce many growth factors and hence transplanting glia to the injury site is an even more promising approach as these cells can integrate with endogenous cells and scar tissue, producing a more long-term growth-promoting environment (Yan et al. 2001; Feron et al. 2005; Cao et al. 2007; Centenaro et al. 2011). Similarly, pluripotent stem cells can be transplanted to the injury site, potentially resulting in neuronal regeneration and production of glial cells. This method is still experimental, but has resulted in promising functional outcomes in animals (reviewed by Antonic et al. 2013). Further, manipulation of gene expression to block production of growth-inhibitory and toxic molecules has also resulted in some promising functional outcomes (reviewed by Leal-Filho 2011). Overall, however, while these therapeutic interventions have led to some positive outcomes, to date, none have produced a significant functional recovery in humans (Lim and Tow 2007; Leal-Filho 2011).
13.2 Endogenous Glial Cells and Their Role in Spinal Cord Injury
Glial cells are the most abundant cells in the nervous system. They are closely associated with neurons and were previously described simply as supportive nervous tissue. A deeper understanding of glial cell biology, however, has demonstrated that glial cells exhibit a multitude of complex roles and are essential for the development and function of the entire nervous system (Jessen 2006). Glial cells are a heterogeneous population of cells that differ in developmental origin, molecular composition, structure and specific behaviour, and exist together with neurons and other cells in an integrated and co-dependent system (Chung and Barres 2012). Throughout the nervous system, glial cells have crucial roles in axonal extension and guidance, protection against mechanical, chemical and oxidative injury, as well as preservation of the electrical and chemical balance of all neurons (Ndubaku and de Bellard 2008).
Glial cells can be broadly classified as being either central nervous system glia or peripheral nervous system glia. In the mature central nervous system (CNS), there are two major types of glial cells of neural origin; astrocytes and oligodendrocytes. Other types of CNS glial cells exist that originate from non-neuronal precursors; microglia constitute part of the innate immune system and originate from macrophage lineages (Chugani et al. 1991). In the peripheral nervous system (PNS), Schwann cells constitute the main glial cell type, with the exception of the olfactory nervous system, which is populated by specialized glia termed olfactory ensheathing cells (OECs).
Astrocytes play a critical role in the function and homeostasis of the CNS. They are required for the formation and maintenance of the blood-brain barrier, provide support for axonal extension and play an active role in neuronal signalling by exchange of ions and production of neurotransmitters, as well as cell adhesion and synapse signalling molecules (Kriegstein and Gotz 2003). Astrocyte–neuron interactions are known to secure the survival and normal function of neurons (Jessen 2004). Numerous studies have demonstrated that astrocytes play important neuroprotective roles, in neurodegenerative disorders (reviewed by Singh et al. 2011; Cabezas et al. 2012) and they have the ability to promote neuronal survival by protecting against reactive oxygen species and other stressors (Lopez et al. 2007).
After spinal cord injury, astrocytes respond rapidly by migrating to the injury site, where they proliferate and form a compact structure, a glial scar, to preserve the blood-brain barrier, protecting the CNS and maintaining the adequate ionic environment necessary for nerve function. However, the glial scar eventually becomes a physical barrier that stops damaged axons from regenerating and reconnecting (Fig. 13.1) (Leal-Filho 2011). Furthermore, astrocytes respond to neuronal injury by increasing their proliferation and by secreting glycoproteins such as chondroitin sulfate proteoglycans (CSPG), which act to inhibit axon elongation (Table 13.1) (Qiu et al. 2002; Su et al. 2009).
Table 13.1
Glial cell response to spinal cord injury
Type of Glia | Response in spinal cord injury event | Reference |
|---|---|---|
Astrocytes | Removal of toxic chemicals (glutamate). Proliferation and secretion of neuroprotective but growth-inhibitory factors. Formation of glial scar | |
Oligodendrocytes | Massive death due to high glutamate concentrations. Production of glycoproteins with Nogo-receptor affinity that will suppress myelin production | |
Microglia | Initially phagocytosing debris and producing neuroprotective factors. Over time become neurotoxic and growth-inhibitory due to constant activation | |
Schwann cells | Cells de-differentiate to an immature state, lose their myelin sheath conformation and migrate from the periphery into the injury site in the CNS, where they participate in endogenous repair processes by expression of neurotrophic factors |
Oligodendrocytes are morphologically similar to astrocytes, albeit with fewer and smaller branched processes. They play different roles in the modulation of neuronal function as well as the regulation of proliferation , survival and differentiation of neurons (Jauregui-Huerta et al. 2010) The most important role of oligodendrocytes, however, is to myelinate axons. The myelin sheet provides electrical insulation around the nerve fibres, speeding the transmission of electrical signals (Jessen 2004). The myelin layer also protects the axons by creating a “safe chamber”, resembling a growth-promoting channel through which the axon extends. After spinal cord injury, populations of oligodendrocytes are rapidly affected by high levels of glutamate and massive cell death follows. Oligodendrocytes that do survive produce neurite outgrowth inhibitor (Nogo), myelin-associated glycoprotein (MAG), and oligodendrocyte-myelin glycoprotein (OMgp) (Table 13.1), proteins that bind to the Nogo receptor, repressing myelin production and affecting axonal outgrowth and neuronal synapses (Jones et al. 2003). Microglia, which are essentially macrophages present within the CNS, respond to injury by migrating to the injury site, where they phagocytose debris, secrete a range of both pro- and anti-inflammatory cytokines and growth factors which initially have a neuroprotective effect. Over time, however, microglia near and in the injury site respond to the constant prolonged activation by secreting molecules that are growth-inhibitory or toxic, thus repressing axonal regeneration (Chatzipanteli et al. 2002; Pearse et al. 2003; Block and Hong 2005). The majority of the activated microglia transition to the M1 type, which can directly induce neuronal death (Kigerl et al. 2009; Gao et al. 2013). Thus, together with the glial scar, the local environment at a CNS injury site inhibits long-term neuronal extension and regeneration.
The PNS differs dramatically from the CNS in terms of capability to regenerate itself after injury. In contrast to central nerves, peripheral neurons in general regenerate after injury, unless large nerves have been completely severed. Schwann cells play an active role in repair of peripheral damaged nerves as a consequence of their ability to differentiate, migrate, proliferate, secrete growth factors , and produce myelin. Schwann cells are classified as either myelinating or non-myelinating. Myelinating Schwann cells enwrap individual peripheral axons, forming the myelin sheath, whereas the non-myelinating type have metabolic and mechanical support functions (Jessen 2004). After spinal cord injury, Schwann cells migrate from the periphery into the injury site within in the CNS, and participate in endogenous repair processes (Table 13.1). They re-enter the cell cycle, lose their myelinating phenotype and de-differentiate into an immature state, and begin to express trophic factors and cell adhesion molecules that provide a more favourable environment for axon regeneration and extension (Oudega and Xu 2006).
One approach to improve the outcomes after spinal cord injury is to transplant glial cells into the injury site to reduce inflammation , and which will help form a glial bridge across the injury site and thereby promote axon extension. The glial of the PNS system, Schwann cells and OECs, have been trialled in animal models and in humans with various outcomes. The OECs have unique characteristics that may confer an advantage over other glial cell types for transplant therapies.
13.3 The Mammalian Olfactory Nervous System
The mammalian olfactory nervous system is one of the few regions in the CNS in which neurogenesis continuously occurs during the lifetime of the organism (Mackay-Sim and Kittel 1991a, b). The primary sensory neurons of the olfactory system line the dorsal/caudal nasal epithelium and are directly exposed to the environment (Fig. 13.2). The neurons are subjected to attack and destruction by bacterial (St John et al. 2014) and viral pathogens as well as toxins within the air and thus need to be replaced throughout life. Whilst the average life-span of olfactory neurons has not been clearly determined in humans, mouse olfactory neurons generally live for one to three months. Neurons that degenerate are rapidly replaced by new neurons arising from progenitor cells that line in the basal layer of the olfactory mucosa (Mackay-Sim and Kittel 1991b), a process that occurs throughout life (Ramon-Cueto and Santos-Benito 2001).
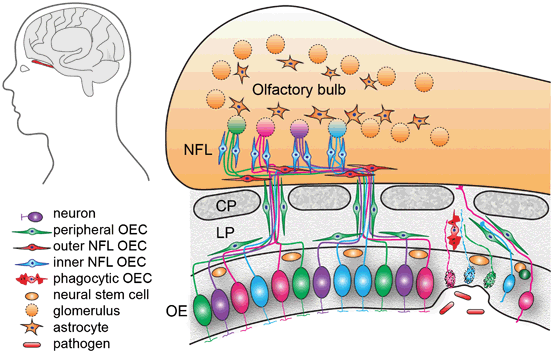
Fig. 13.2
Anatomical organisation of the olfactory system. Primary olfactory sensory neurons lie within the olfactory epithelium (OE). Their axons project through the cribriform plate (CP) and enter the olfactory bulb where they terminate in their target glomeruli. OECs within the lamina propria (LP) encase the bundles of numerous different axons as they project to the olfactory bulb. In the outer nerve fibre layer (NFL) of the olfactory bulb, the OECs (red) aid the defasciculation and sorting of the different axons. In the inner layer of the nerve fibre layer the OECs (blue) assist with the refasciculation and targeting of similar axons to their targets. Astrocytes form a barrier around the glomeruli (dashed circles). The olfactory sensory neurons within the OE are subjected to toxic molecules within the inhaled air and pathogens such as bacteria and viruses which can result in the death of the neurons (spotted neurons). OECs phagocytose the debris from the degenerated axons. Stem cells lining the basal layer of the OE replenish the neuron population which project axons through channels maintained by the OECs
The primary olfactory system comprises the olfactory mucosa and the bundles of olfactory nerves that project into the olfactory bulb. Stem cells that line basal layer of the olfactory epithelium give rise to the primary olfactory sensory neurons which migrate apically to populate the olfactory epithelium (Fig. 13.2). Olfactory sensory neurons have a bipolar morphology with a single dendrite extending onto the surface of the epithelium and a single axon projecting to and terminating in the olfactory bulb. Each olfactory neuron expresses a single odorant receptor type with the neurons mosaically distributed throughout the epithelium, but the axons of the same odorant receptor type converge to the same targets within the olfactory bulb (Vassar et al. 1994; Mombaerts et al. 1996). To reach their targets in the olfactory bulb, the axons of the olfactory sensory neurons project through the lamina propria that underlies the olfactory epithelium and pass through the bony cribriform plate to enter the nerve fibre layer which is the outer layer of the olfactory bulb and within the CNS. Thus, new axons must constantly traverse the PNS-CNS border and find their correct targets inside the olfactory bulb (Valverde et al. 1992; Tennent and Chuah 1996; Chehrehasa et al. 2010). The constant ability of olfactory neurons to regenerate and the unique ability of olfactory axons to extend across the PNS-CNS boundary are attributed to the presence of the glia of the olfactory system, called OECs.
13.3.1 Olfactory Ensheathing Cells—the Glia of the Olfactory System
OECs arise from neural crest (Barraud et al. 2010) and they are constantly in close contact with the axons of olfactory neurons all the way from the nasal epithelium to the outer layer of the olfactory bulb. OECs ensheathe the axons of olfactory neurons by the extension of cytoplasmic processes (Chuah and Zheng 1992; Tennent and Chuah 1996) followed by the fasciculation of the axons into larger bundles which ultimately join to form the olfactory nerve (Whitesides and LaMantia 1996). In contrast to Schwann cells , which in the process of myelination enwrap one single axon (Fig. 13.3), OECs ensheathe bundles of multiple axons by projecting extensive thin cytoplasmic processes around and between the numerous axons within the fascicles (Fig. 13.2; 13.3).
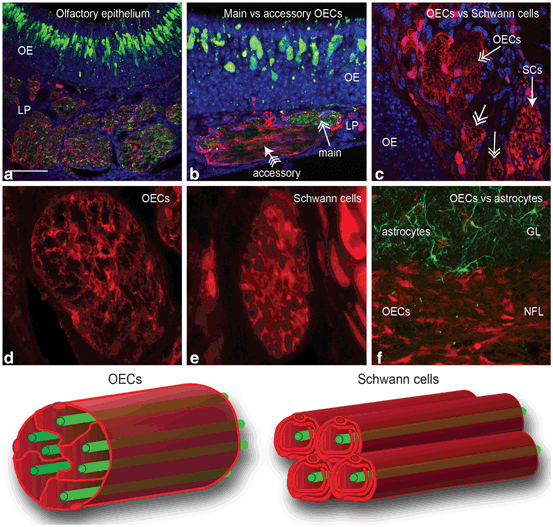
Fig. 13.3
The different populations of glia in the olfactory system. Panels a-f show coronal sections through the olfactory system of a transgenic reporter mouse (OMP-ZsGreen X S100ß-DsRed; Windus et al. 2007; Ekberg et al. 2011) that expresses ZsGreen fluorescent protein in olfactory neurons and DsRed fluorescent protein in glia. a The primary olfactory neurons (green) within the olfactory epithelium (OE) project axons into the lamina propria (LP) where they coalesce into fascicles wrapped up by OECs (red). Nuclei (blue) are stained with DAPI. b Along parts of the septum in the nasal cavity, the axon fascicles of the main olfactory system are adjacent to axon fascicles of the accessory (pheromone) olfactory system. c Branches of the trigeminal nerve also innervate the nasal cavity; the trigeminal nerve axons are encased by Schwann cells with the trigeminal nerves running adjacent to the main olfactory nerve fascicles that are encased by OECs (double-headed arrows). d The cell bodies of the olfactory glia are mainly restricted to the periphery of the axon fascicles with their processes permeating the central regions of the axon fascicle. e Schwann cells of the trigeminal nerve form tube-like encasing of individual axons. f In the olfactory bulb, OECs (red) in the nerve fibre layer (NFL) form a barrier with the astrocytes (green; GFAP immunostaining) in the glomerular layer (GL). g Schematic of the ensheathment of olfactory axons by OECs. The cell bodies of OECs are largely restricted to the exterior and the processes of the OECs penetrate the internal areas of the nerve bundle where they surround numerous olfactory axons. h Schematic of Schwann cell ensheathment of other peripheral nerves in which individual axons are myelinated and encased by Schwann cells. Scale bar is 65 μm in a; 50 μm in b; 30 μm in c; 20 μm in d; 15 μm in e; 40 μm in f
OECs also have a role in promoting axon growth and are known to secrete numerous axon growth promoting factors, such as nerve growth factor, brain derived neurotrophic factor and neuregulins (Boruch et al. 2001). During development, OECs proliferate and migrate ahead of axons or surround the growth cones of axons (Tennent and Chuah 1996; Chehrehasa et al. 2010). Loss of OECs from the olfactory nerve during development results in poor axon growth and targeting (Barraud et al. 2013) which demonstrates that OECs are crucial to the growth and maintenance of axons.
OECs are also thought to be crucial for regeneration during normal turnover of olfactory sensory neurons or after large-scale infection by bacteria and viruses, or major injury. Bacterial infection can lead to the death of olfactory sensory neurons and subsequently their axons (Fig. 13.2; St John et al. 2014), or injury can directly lead to the destruction of the axons (Graziadei et al. 1978; Chehrehasa et al. 2010). The debris from the degenerated axons must be removed but unlike other areas of the body where cells of the immune system usually clear away debris, in the olfactory system this function primarily relies on the OECs (Su et al. 2013). OECs have been shown to continuously phagocytose debris arising from the degenerating axons that occurs during normal turnover of neurons or after widespread injury (Wewetzer et al. 2005; Su et al. 2013). OECs are also able to phagocytose bacteria and thereby protect the olfactory pathway from infection (Wewetzer et al. 2005; Leung et al. 2008; Panni et al. 2013).
OECs form a three-dimensional structure resembling a tunnel through which the axons extend (Fig. 13.3; Li et al. 2005). These structures remain intact even after olfactory axons have degenerated completely following large-scale injury to the olfactory epithelium (Li et al. 2005). However, after large scale injury, OECs can proliferate not only locally around the injury but also from precursors that are present in the olfactory mucosa after which they then migrate along the olfactory nerve (Chehrehasa et al. 2012). By maintaining open channels through which regenerating axons can extend and by responding to injury by proliferating and migrating to the region of need, the OECs with their axon growth-promoting properties provide the structure and support needed for the continuous successful regeneration of the olfactory system.
OECs produce numerous growth factors such as fibroblast growth factor (FGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), as well as neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), neurotrophin 4 (NT4) and NT5; as well as extracellular matrix and cell adhesion molecules including laminin, collagen, galectin-1, heparin sulfate proteoglycans, glial-derived nexin and N-cadherin (Doucette 1990; Doucette and Devon 1993; Chuah and Teague 1999; Kafitz and Greer 1999; Tisay and Key 1999; Boruch et al. 2001; Woodhall et al. 2001; Woodhall et al. 2003; Chuah et al. 2004; Chung et al. 2004; Vincent et al. 2005b; Mackay-Sim and St John 2011). These OEC-derived factors are likely to play an important role in nerve repair and regeneration processes as well as neutralization of toxic cell environments due to the excess of free radicals and neurotransmitters such as glutamate (Doucette 1995; Ramon-Cueto 2000; Woodhall et al. 2001; Woodhall et al. 2003; Ramon-Cueto 2011).
13.3.2 Differences Between Olfactory Ensheathing Cells and Schwann Cells
Originally, OECs were referred to as Schwann cells of the olfactory system (Doucette 1984), but their distinctive characteristics separated them from other glial cell types to such an extent that they were classified as an individual glial type. OECs possess features of both CNS and PNS glia in terms of morphology and molecular profile, consistent with their location to both the central and peripheral part of the olfactory nervous system and their ability to cross the PNS-CNS interface. Developmentally, OECs and Schwann cells are of neural crest origin (Barraud et al. 2010), in contrast to astrocytes, which arise from radial glia of neuroepithelial origin (Kriegstein and Gotz 2003). OECs are known to express a number of different proteins found in either Schwann cells or astrocytes. For example non-myelinating Schwann cells and OECs (except those in the inner nerve fibre layer of the olfactory bulb) present immunoreactivity for the p75 low-affinity neurotrophin receptor (p75NTR) (Ramon-Cueto 2000).
Whilst similarities between Schwann cells and OECs are evident, one particularly important difference exists in the ability to interact with astrocytes . In contrast to Schwann cells , OECs interact freely with astrocytes, without causing detrimental effect on the astrocyte population (Lakatos et al. 2000). This specific feature is of great interest for nerve regeneration therapies where both populations (OECs and astrocytes) interact at an injury site (Chuah et al. 2011). When OECs are confronted with astrocytes in spinal cord injury sites, astrocyte processes, which form the glial scar, alter their morphology to create a bridging pathway with OECs that allow severed axons to extend across the lesion establishing functional connections (Ramer et al. 2004; Li et al. 2012).
In contrast to Schwann cells, OECs migrate ahead of the regenerating axons, extending their processes to provide a cellular pathway that facilitate axonal extension and adhesion (Tennent and Chuah 1996; Chehrehasa et al. 2010). OECs increase their migration ability by the formation of bigger and thicker processes (Valverde et al. 1992), maintaining a continuous ensheathment of the axons during the regeneration process and leading to enhanced axon growth (Chehrehasa et al. 2010). The capacity of OECs to promote olfactory system renewal and regeneration, as well as their capacity to bridge, enter, and interact with cells of injured host tissue, constitute key factors contributing to the increasing interest in the use of transplanted OECs as therapeutic candidates in spinal cord injury treatments.
13.4 Use of Glial Cells in the Treatment of Spinal Cord Injuries
Re-establishment of nerve connections after spinal cord injury depends of the ability of axons to extend along a pathway to reach their targets. This living pathway consists of glial cells, which provide a dynamic channel through which axons can extend towards their targets (Ramer et al. 2004; Li et al. 2012). After spinal cord injury, the severed nerves are able to survive and sprout locally. However, they are unable to elongate and re-establish the connections, primarily because the glial pathway is altered, blocked and sometimes completely lost. Consequently, a primary objective in the treatment of spinal cord injury is re-establishment of the glial pathway. Transplantation of glial cells into the injury site is therefore a promising therapeutic approach for repair spinal cord injury (Oudega and Xu 2006).
Glial cell transplantation addresses many of the challenges that must be overcome for successful functional improvement, including (1) re-establishment of a growth-promoting environment, (2) replacement of lost cell populations (neurons and glia) , and (3) facilitation and promotion of axonal regeneration and extension. Pioneering studies have established that transplantation of glial cells can improve axonal repair, enhance re-growth of damaged nerve cells and improve functional recovery (Yan et al. 2001; Santos-Benito and Ramon-Cueto 2003). Additionally, glial cells have the potential to produce neurotrophic molecules that activate axon regeneration and extension (Jones et al. 2003; Feron et al. 2005).
Different types of glial cells have been investigated as treatment for spinal cord injury including Schwann cells from peripheral nerves and OECs. Schwann cells have been trialled for transplantation due to the important role they play in axon regeneration and myelination. Schwann cells transplanted to the damaged spinal cord can stimulate regeneration of damaged neurons, presumably due to the production of neurotrophic factors (Park et al. 2010), and can also enhance axon remyelination and extension (Lavdas et al. 2010; Flora et al. 2013). However, the axonal regeneration has thus far been limited to restricted areas because Schwann cells have failed to migrate considerable distances into the injured tissue (Lankford et al. 2008). The limited migration is most likely due to unfavorable interaction between the transplanted Schwann cells and host astrocytes (Li et al. 2012). Additionally, Schwann cells have been reported to inhibit myelination by the secretion of connective tissue growth factor, whereas OECs do not (Lamond and Barnett 2013). Thus, Schwann cells may not be the optimal cell type for transplantation therapies due to their poor migration properties, inability to freely interact with endogenous glia and expression of inhibitory molecules.
13.4.1 Transplanted OECs in Spinal Injury Models
Implantation of OECs to promote repair after spinal cord injury have been performed in a variety of spinal cord injury animal models (Table 13.2). Most of these studies have shown that in rodents with spinal cord injury, OEC transplantation promotes the regeneration of axons (Bartolomei and Greer 2000; Gudino-Cabrera et al. 2000; Ramer et al. 2004) and improves functional restoration of breathing and climbing ability (Li et al. 2003; Su and He 2010; Stamegna et al. 2011). In a study of spinal-injured dogs, the transplantation of OECs and fibroblasts restored significant movement in some dogs probably through the restoration of local circuitry, which clearly demonstrates that the procedure has high potential (Granger et al. 2012). Some studies, however, concluded that transplantation of OECs into injured spinal tract did not lead to any detectable difference in axonal extension and functional outcomes (Collazos-Castro et al. 2005; Lu et al. 2006; Chhabra et al. 2009). Discrepancies between results may be attributed to different variables such as the exact nature of spinal cord injury, OEC isolation and culture protocols, transplantation procedure (site of the OECS transplantation and the time after injury that the transplantation is performed) and methodology used to assess outcome.
Table 13.2
Recent examples of in vivo transplantation of olfactory ensheathing cells
First Author (year) | Species | Transplanted cells/tissue | Purity of OECs | Main Outcomes |
|---|---|---|---|---|
Richter (2005) | Mouse | Lamina propria Olfactory Bulb | 92 % p75 positive cells | Stimulation of outgrowth of axon sprouting. Enhanced angiogenesis LP derived OECs superior ability to migrate |
Collazos-Castro (2005) | Rats | Olfactory Bulb derived OECs | 90 % p75 positive cells | Partial improvement of motor function. No improvement in axonal regeneration |
Human | Lamina propria derived OECs | 76–88 % p75 positive cells 95 % S100 and GFAP positive cells | Transplantation is feasible and is safe up to 3 years of post-implantation. No deterioration in neurological or functional level | |
Lu (2006) | Rats | Lamina propria derived OECs | 97 % p75 positive cells | Partial Improvement on axonal regeneration. No difference compared with fibroblast |
Huang (2006) | Human embryos | Olfactory bulb derived cells | n/a | Neurological functional improvement after transplantation |
Lima (2006) | Humans | Whole layer olfactory mucosa | n/a | Transplantation is feasible, relatively safe, and potentially beneficial. Recovery of bladder sensation and improvement in motor function scores |
Toft (2007) | Rats | Olfactory Bulb | 98 % p75 Positive cells | Improvement on spinal cord function in sensory pathways |
Yamamoto (2009) | Rats | Olfactory mucosa | Mixed culture (5 % p75 positive) | Restored directed fore-paw retrieval but not axon regeneration observed |
Munoz-Quiles (2009) | Rats | Olfactory Bulb | n/a | Progressive improvement in motor function and axonal regeneration |
Salehi (2009) | Rats | Embryonic stem cells + Olfactory Bulb OECs | 95 % p75 positive cells | Neural regeneration, neuron survival and partial functional recovery |
Chhabra (2009) | Humans | Whole layer olfactory mucosa | n/a | Procedure is relatively safe and feasible. No efficacy could be demonstrated |
Aoki (2010) | Rats | Whole layer olfactory mucosa | n/a | Partial improvement in motor function and axonal regeneration |
Ma (2010) | Rats | neurotrophin-3 genetically modified Olfactory bulb derived OECs | 95 % p75 and S100ß positive cells | Effective improvement of axonal regeneration and motor function OECs able to produce NT-3 in vivo |
Amemori (2010) | Rats | Lamina propria derived OECs + cAMP infusion | 75 % p75 positive cells | Improvement motor function Axon regeneration, sprouting and branching Reduce astrocytic hypertrophy |
Ziegler (2011) | Rats | Olfactory bulb | n/a p75 Immuno purification | Improvement in hind limb function, injection of OEG facilitated regeneration of axons across a complete mid-thoracic spinal cord transection. |
Centenaro (2011) | Rats | Lamina propria derived OECs | n/a | Discrete motor improvement, improved tissue sparing and sprouting |
Zhang (2011) | Rats | Lamina propria derived OECs | n/a | Disappearance of the lesion cavity and integration of repaired tissue Activation of host Schwann cells, improved myelination |
Novikova (2011) | Rats | Olfactory bulb derived OECs | 93–95 % | Improvement in motor function and neuronal regeneration Aged cell are less effective |
Tharion (2011) | Rats | Olfactory lamina propria derived OECS + Olfactory Nerve Fibroblasts (ONL) | Around 50 % p75 positive cells + 50 % fibronectin positive cells (ONL) | Improvement in diaphragm activities Motor function and axonal regeneration improved Disappearance of the lesion cavity |
Stamegna (2011) | Rats | Lamina propria derived OECs | 90 % p75 positive cells | Improvement in axonal regeneration and motor function. Improvement in diaphragm and phrenic nerve activities |
Granger (2012) | Dogs | Olfactory mucosa | 50 % p75 positive cells | Significantly better fore–hind coordination. Effects are likely to be on local intraspinal circuitry |
Huang (2012) | Human embryos | Olfactory bulb derived cells | n/a | Treatment feasible after more than 3 years. Improvement of neurological function. No adverse effects noted |
Tabakow (2013) | Humans | Olfactory mucosa | 20–50 % S100 positive cells | Improvement of neurological function observed. No adverse effects noted |
Mayeur (2013) | Rats | Lamina propria and olfactory bulb | > 97 % p75 | Improved axon regrowth and reduced scar formation |
Toft (2013) | Rats | Olfactory bulb | > 97 % | Reduced astrocytic hypertrophy |
Torres-Espin (2014) | Rats | Olfactory bulb derived cells | 75 % p75/S100 positive cells | Protection/preservation of tissue around injury site |
Numerous studies have reported significant functional improvements following transplantation of OECs. In rodents, OEC transplantation improved or preserved local circuitry as detected electrophysiologically (Toft et al. 2007), and has improved fore-paw movement (Yamamoto et al. 2009), motor function (Munoz-Quiles et al. 2009; Centenaro et al. 2011; Stamegna et al. 2011), hindlimb function (Salehi et al. 2009; Amemori et al. 2010; Aoki et al. 2010; Ziegler et al. 2011), plantar function (Amemori et al. 2010), locomotor function (Ma et al. 2010) and diaphragm activity (Tharion et al. 2011). Thus depending on the type of injury and the assessment of particular functional tests, OEC transplantation can improve functional outcomes.
Corresponding anatomical outcomes after OEC transplantation have also been detected with the majority of studies focusing on axon growth and reduction in cavity formation and astrocytic scarring (Amemori et al. 2010; Centenaro et al. 2011; Tharion et al. 2011; Zhang et al. 2011; Toft et al. 2013; Torres-Espin et al. 2014). The reduction in cavity formation and astrocytic scarring are considered important features as they preserve the axonal circuitry and reduce the formation of physical barriers that prevent axonal growth. In these situations, OECs could be considered to indirectly promoting axon growth as they are enhancing the local environment to make it subsequently amenable to axon growth across the injury site.
However, anatomical improvements are not always detected despite functional recovery being observed. Significant restoration of fore-paw retrieval but not axon regeneration (Yamamoto et al. 2009) may indicate local circuitry changes outside of the immediate injury site as has been suggested for restoration of function in dogs (Granger et al. 2012) or may indicate improved preservation of function of the spared axons.
The timing of transplantation of OECs may also be important for anatomical and functional outcomes. Acute transplantation of OECs may be preferable as the potential ability of OECs to reduce cavity formation and reduce the creation of an inhibitory environment would likely promote axon regeneration. Indeed, acute transplantation of OECs has shown higher functional and anatomical outcomes compared to delayed transplant (Lopez-Vales et al. 2006). However, other studies have not detected significant differences in outcomes for acute versus delayed transplantation of OECs (Munoz-Quiles et al. 2009; Centenaro et al. 2011; Torres-Espin et al. 2014). Despite this, it would seem reasonable that differences in efficacy and outcomes of acute versus delayed treatment may be revealed if improvements in the experimental conditions were made and the purity of the cell preparations and co-transplanted cells was optimized (as discussed below).
What are the mechanisms by which OECs promote regeneration of the spinal cord? OECs transplanted into spinal cord lesions promote regeneration of axons across the lesion, remyelination of axons (although the mechanism remains unknown) and resumption of numerous cellular functions (Ramon-Cueto 2000; Santos-Benito and Ramon-Cueto 2003; Ramer et al. 2004; Li et al. 2012). In particular, OECs provide a physical “bridge” which assists directional regeneration of severed axons across injury sites (Ramon-Cueto and Nieto-Sampedro 1994; Boruch et al. 2001; Resnick et al. 2003) which is consistent with the role of OECs within the injured olfactory system where it has been shown that OECs migrate ahead of axons and that superior axon regeneration occurs when a substantial OEC environment has been created (Chehrehasa et al. 2010). Different factors such as GDNF and NGF have been identified to regulate migration of OECs (Cao et al. 2004; Windus et al. 2007). For example, when OECS are genetically modified to express higher levels of GDNF they display a superior ability to promote axonal growth (Cao et al. 2004). On a cellular level, GDNF acts by stimulating the behaviour of the peripheral lamellipodial waves, increasing cell-cell contact and contact-dependent migration (Windus et al. 2007). Indeed, it has been demonstrated that the motility of OECs in vitro is directly responsible for the apparent motility of axons due to the preference for axons to adhere to OECs (Windus et al. 2011). Thus the migration of OECs across the spinal cord injury site creates a physical glial bridge rich in axon growth promoting factors which together is likely to enhance axon regeneration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








