Fig. 3.1
Regulatory history and perspectives on SIB assessment. a 1991–2008 b 2009–2013
European Regulators were the first to post restrictions on the use of antidepressants. In 2003, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) initiated urgent safety restrictions to contraindicate the use of selective serotonin reuptake inhibitor (SSRI) antidepressants, with the exception of fluoxetine, in children and adolescents. This had followed comprehensive reviews of pediatric depression clinical trial data by the Committee on Safety of Medicines (CSM). The MHRA stated that “on the basis of this review of the available clinical trial data, CSM has advised that the balance of risks and benefits for the treatment of major depressive disorder (MDD) in under 18s is judged to be unfavorable for sertraline, citalopram, and escitalopram, and is unassessable for fluvoxamine. Only fluoxetine (Prozac) has been shown in clinical trials to have a favorable balance of risks and benefits for the treatment of MDD in the under 18s” (MHRA 2003).
Subsequently in 2004, after lengthy analysis and review of clinical trial data, FDA required that labeling of specific antidepressants carry black box warnings, intended to alert healthcare providers and patients to increase monitoring of troubling symptoms. At the time of these regulatory actions, many public health and mental health professionals (MHPs) were concerned that deterring the prescription of antidepressants might lead to undertreatment of depression and in turn to an increase in suicide rates for untreated MDD. Two published studies highlighted this concern, indicating that between 2003 and 2005 in the US there was a 20 % reduction in prescription of antidepressants and, at the same time from 2003 to 2004, an increase in youth suicide rate by 14 % (Gibbons et al. 2007) and new diagnoses of depression in pediatric populations dropped by 44 % (Libby et al. 2009). In addition, previous pharmacoepidemiologic studies had reported that adolescent suicide rates had declined in the early 1990s, related to increasing use of antidepressant drugs in this population (Fergusson et al. 2000, 2005; Gibbons et al. 2006; Olfson et al. 2003; Sondergard et al. 2006; Simon 2006; Valuck et al. 2007).
Subsequent to the regulatory reviews of antidepressant medication, TESI was the focus of further analyses for other classes of centrally acting drugs, particularly anticonvulsants, antipsychotics, and anxiolytics. These analyses resulted in a number of regulatory labeling changes and/or risk management actions as a result (see Table 3.1 for further details). Risk management actions typically focus on ensuring appropriate warnings and wording are reflected in patient materials, such as medication guides, as well as the physician prescribing information. Not all warning language in the product information includes both SIB. For example, product information for Strattera (atomoxetine) described suicidal ideation risks. While a number of the regulatory analyses and reviews were conducted as class reviews (e.g., antidepressants, antiepileptic drugs), not all assessments of SIB relied on data from across compounds (e.g., Strattera is the only ADHD medication with specific warning language for suicidal ideation). A consistent feature across the product labeling for compounds (Table 3.1) is the inclusion of other psychiatric symptoms that may be associated with increased risk of SIB, including mood disturbances, anxiety and/or disturbing thoughts. In addition, recent labeling inclusions for antidepressants have referenced a lowering of suicidal risk in the >65-year age group, based on reviews showing lowered relative risk of events in this age group.
Table 3.1
Summary of labeling changes for FDA reviews of different medication classes for suicidal ideation and behavior (as of March 2011)
Drug class | Drugs | Class labeling | Black box | Warnings | REMS | Med guide | Comments |
|---|---|---|---|---|---|---|---|
Antidepressants | √ | √ | √ | √ | Black box—suicidality and antidepressants | ||
Children, adolescents, young adults | |||||||
Reduction in risk in 65+ years | |||||||
Antiepileptics | √ | √ | √a | Warnings and precautions—suicidal behavior or ideation; antiepileptic drugs increase the risk of suicidal thoughts | |||
ADHD | |||||||
Strattera | √ | √ | √ | Black box—suicidal ideation: children or adolescents | |||
Wording also in patient counseling info | |||||||
other stimulants | √ | No focused language for suicidality | |||||
Antipsychotics (depression) | |||||||
Abilify, Seroquel | √ | √ | √ | Black box—suicidal thinking and behavior: children, adolescents, young adults | |||
Smoking cessation | |||||||
Chantix, Zyban | √ | √ | √ | √ | Black box—suicide ideation, suicide attempt and completed suicide reported in patients | ||
Leukotriene inhibitors | |||||||
Singulair | √ | Suicidal thinking and behavior (including suicide) | |||||
Accolate, Zyflo | Warnings for neuropsychiatric events not suicide | ||||||
Sedative/hypnotics | |||||||
Lunesta | √ | √ | √ | Suicidal thoughts and actions (including completed suicides) | |||
Also in dosing section | |||||||
Ambien | √ | √ | √ | Suicidal thoughts and actions (including completed suicides) | |||
Warning in special populations (depressed patients) | |||||||
Ativan | √ | Precaution and AE section | |||||
Acne | |||||||
Accutane | √ | Suicidal ideation, suicide attempts, suicide | |||||
Patient informed consent for suicidal thoughts, suicide attempts, suicide | |||||||
Other indications | |||||||
GERD | Reglan | √ | √ | Based on post-marketing events | |||
Warnings/CNS effects: suicidal ideation and suicide | |||||||
Huntington’s disease | Xenazine | √ | √ | √ | Clinical trial events including completed suicide | ||
Multiple sclerosis | Interferons | √ | √ | Suicidality based on post-marketing events | |||
3.3 US Regulatory Guidance on SIB
The result of the regulatory focus on SIB is the need to implement prospective assessment for treatment emergent SIB in clinical trials for CNS-active medicines. In addition, the same assessment tools can be used to measure prior history of suicidal ideation or behavior, and the implementation of other psychiatric screening tools are helpful to elucidate current or history of psychiatric comorbidities.
The FDA’s first draft guidance document, entitled “Suicidality: Prospective Assessment of Occurrence in Clinical Trials” was issued in September 2010. The main rationale for this document was to “Ensure patients who are experiencing suicidality are properly recognized and adequately treated.” In addition, the guidance was intended to “Ensure collection of more timely and more complete data on suicidality to better detect increased suicidality in individual studies and in pooled analyses. This is important whether or not a particular drug is known to be associated with treatment-emergent SIB.” Subsequently, in August 2012, the FDA issued a revised draft guidance document, entitled “SIB: Prospective Assessment of Occurrence in Clinical Trials”. The revised FDA guidance reflects the FDA’s current thinking on the importance of assessment of SIB in psychiatric and nonpsychiatric drug trials conducted under Investigational New Drugs (INDs) and general principles for how best to accomplish SIB assessment in drug development.
The major revisions in the FDA’s revised draft guidance of August 2012 include the following:
The term suicidality is replaced with the phrase suicidal ideation and behavior.
An expanded set of the C-CASA categories is provided, along with definitions and explanations.
The advice on particular trials and patients that would need assessments of SIB and the timing of such assessments is revised.
Concerns about the time burden of assessments are addressed.
Questions about the possible value of the assessments providing protection for patients in the trials themselves are discussed.
It is made clear that use of an assessment instrument that directly classifies relevant thoughts and behaviors into C-CASA categories eliminates the need for any additional coding.
Additional advice on evaluation of alternative instruments is provided.
The scope of the revised FDA draft guidance encompasses both psychiatric and neurologic drugs as well as drugs for nonpsychiatric indications (e.g., isotretinoins, other tretinoins, beta blockers, reserpine, smoking cessation drugs, and drugs for weight loss). The revised FDA draft guidance was adopted by the Division of Psychiatry Products, and the Division of Neurologic Products. It is anticipated that the draft guidance will become the standard approach for other FDA divisions as well [such as the Division of Pulmonary, Allergy and Rheumatology Products (with regard, especially, to leukotriene-modifying drugs)]. It is unclear when the 2012 draft guidance will be finalized.
3.4 The Columbia Suicide Severity Rating Scale
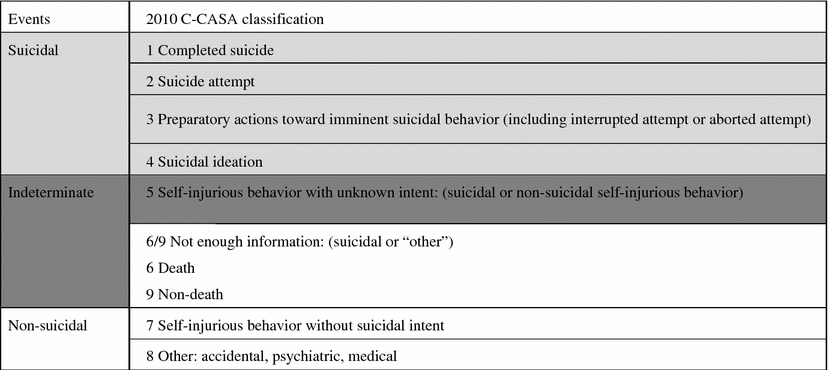
The 2010 draft FDA guidance recommended the use of a particular SIB assessment, the C-SSRS. This instrument was designed to be used prospectively in clinical trials and was intended to systematically ascertain and document the occurrence of suicidal events. These events were defined as indicative of SIB based on the use of the retrospective tool, the C-CASA (Posner 2009; see Table 3.2). The C-CASA was developed by Kelly Posner and her team at Columbia University. C-CASA provided a common language to classify SIB data derived from retrospective examination of clinical trial submitted to FDA.
Table 3.2
C-CASA classification scheme from 2010 FDA guidance
 |
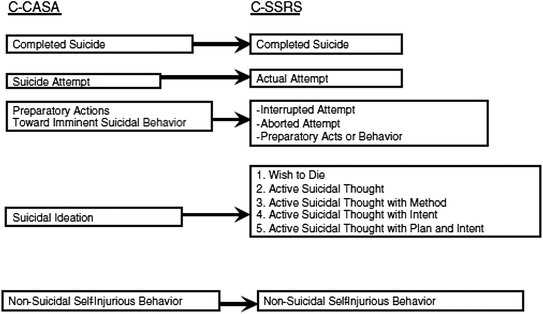
C-CASA is the retrospective counterpart of the more detailed prospective classification instrument, the C-SSRS. It contains a 1–5 numerical rating scale for suicidal ideation of increasing severity (from a “wish list to die” to an “active thought of killing oneself with plan and intent”). By contrast, the C-CASA in the 2010 FDA guidance only had one ideation item (classification 4). How the C-CASA and C-SSRS mapping is done is shown in Fig. 3.2.
The 2012 revision continues to emphasize the use of the C-SSRS and highlights that it classifies SIB events directly to the 11 expanded C-CASA categories without the need for additional narratives or coding. This direct classification of events is viewed by the FDA as one of the C-SSRS’s strengths.
The standard adopted by the FDA for coding, summarizing and analyzing SIB data in the 2012 revision of the draft guidance is an expanded version of the C-CASA. This version has 11 categories in total: 5 for suicidal ideation, 5 for suicidal behavior, and 1 for self-injurious behavior with no suicidal intent. Data collected for assessment (whether retrospectively or prospectively) must be classified into these 11 categories as defined by the FDA (see Table 3.3 for a listing of the categories).
Table 3.3
Revised guideline (2012) suicidal ideation and behavior events and categoriesa
Category | Event |
|---|---|
Suicidal ideation | |
1 | Passive |
2 | Active: nonspecific (no method, intent, or plan) |
3 | Active: method, but no intent or plan |
4 | Active: method and intent, but no plan |
5 | Active: method, intent, and plan |
Suicidal behavior | |
1 | Completed suicide |
2 | Suicide attempt |
3 | Interrupted attempt |
4 | Aborted attempt |
5 | Preparatory actions toward imminent suicidal behaviors |
Self-injurious behavior, no suicidal intent | Self-injurious behavior, no suicidal intent |
The revised draft FDA guidance does state that other appropriate prospective SIB assessments can be used and instructs sponsors to discuss proposed alternatives with the relevant review division prior to implementing them. For example, the FDA’s view of the Sheehan Suicide Tracking Scale (S-STS) and in particular its ability to classify SIB events into the 11 expanded C-CASA categories, is not clear at this time.
3.5 European Regulatory Guidance
Unlike the FDA, the European Medicines Agency (EMA) has not yet produced a guidance document specific to assessing SIB in clinical trials. However, in 2010, the EMA identified SIB as a priority in a “2010 Priorities for Drug Safety Research” paper calling for increased research into suicide research methodologies (Issued 4 August 2009). Of particular interest in this paper, are methods to separate suicide associated with certain diseases from treatment for those diseases. In addition, SIB was incorporated into disease specific guidances as part of underlying disease as well as the risk assessment thereof. Disease-specific guidances that encompass suicidal ideation and behaviour include those for: Alcohol dependence, Attention Deficit Hyperactivity Disorder, Depression, Epileptic Disorders, Multiple Sclerosis, Obsessive Compulsive Disorder, Panic Disorder, Post Traumatic Stress Disorder, and Treatment of Smoking. The most recently issued guidances include a statement that C-CASA is an available tool that can be used in these assessments, although there is no explicit expectation to use this tool. Many European clinical trial investigators are familiar with use of the MINI neuropsychiatric interview for assessment of suicide risk at baseline, and have used depression scale suicide-specific items/questions for detection of suicidal symptoms. There is growing familiarity with the use of C-SSRS in global clinical trials, although the construct of the scale is still thought to be very US-centric. Product labels in Europe incorporate similar warning language in the Special Precautions and Warnings for Use section of the Summary of Product Characteristics for all of the products with suicidality warning in the US Product Information.
3.6 Impact of FDA Guidance on Clinical Trials
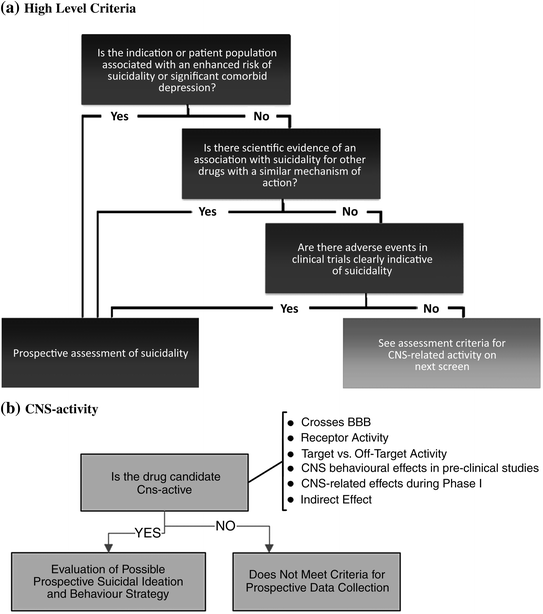
For new drugs undergoing clinical development, assessment of SIB is incorporated into benefit/risk decision-making and continuing risk management approaches throughout the pharmaceutical industry and academia. A number of companies have developed internal guidances, which may include quantitative decision-criteria (i.e., based on binding data at CNS targets) or qualitative clinical judgment (i.e., based on mechanistic understanding and emerging safety profiles) of drug candidates that may require the inclusion of prospective SIB tools. In this section, we will outline a number of structured qualitative steps that are common throughout most large pharmaceutical companies.
Based on the 2012 revision of the FDA guidance, trials conducted under an IND in the following specific categories should address plans for SIB monitoring:
CNS Drug Candidates:
Prospective SIB assessments should be carried out in all inpatient and outpatient clinical trials involving any drug being developed for a psychiatric indication (i.e., those indications managed in the Division of Psychiatry Products), as well as for all antiepileptic drugs and other neurologic drugs with CNS activity. These trials include multiple-dose Phase 1 trials involving healthy volunteers. Questions on what constitutes CNS activity can be directed to the Division of Neurology Products, although some companies make this decision based on CNS clinical signs in preclinical studies, evidence of or expected brain penetration, as well as functional pharmacological data on typical CNS target receptors, ion channels, or transporters (typically through in vitro profiling).
Non-CNS drug Candidates:
Although prospective SIB assessments are not required for compounds/drugs that do not have overt CNS effects, there are some types of trials in this category for which the draft FDA guidance recommends that prospective SIB assessments be performed. This includes all clinical trials for drugs that are pharmacologically similar to drugs where possible signals of risk for SIB have been identified, including isotretinoin and other tretinoins, beta blockers (especially those entering the brain), reserpine, drugs for smoking cessation, and drugs for weight loss.
Leukotriene-modifying drugs are not explicitly discussed in the August 2012 Guidance; however, emerging regulatory intelligence for similar agents suggests that there is an expectation by the Division of Pulmonary, Allergy, and Rheumatology Products for inclusion of SIB assessment with agents acting in the leukotriene pathway.
In addition to mechanistic assessment of new treatments under development, the underlying disease under study and patient population specific considerations need to be taken into account when evaluating prospective SIB assessment. In particular, if the intended indication is one in which the background rate of SIB is considered to be elevated compared with the general population, or if patients have overlapping comorbidity with mood and/or anxiety disorders, then prospective assessment of SIB is recommended. Figure 3.3 outlines a high level structured decision tree for evaluation of SIB in clinical trials.