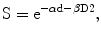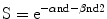Fig. 31.1
CyberKnife Stereotactic Radiosurgery utilizes a noninvasive technique of imaging the patient, including lesion and critical structures, with several x-ray cameras to deliver the radiation dose precisely to the targeted lesion volume in real-time. (a) Three-dimensional model of contoured cervical lesion, highlighting radiosensitive structures such as spinal cord, esophagus, and vagus nerve. (b, c) Lesion is contoured in transverse, coronal, and sagittal planes. (d) Non-isocentric treatment delivery typically results in highly conformal coverage and rapid dose fall-off outside the target
The technique for planning and contouring has been described (Degen et al. 2005). Patients are generally placed supine in a vacuum-bag immobilizer with head and knee support. Thin-sliced CT scans (1.25-mm slice thickness and spacing), centered on the target, are used for contouring and treatment planning. In some cases, image fusion with MRI or PET is performed to assist in target delineation. Contouring is performed by the neurosurgeon. The volume targeted for treatment with the prescription dose is the clinical treatment volume (CTV). The CTV encompasses the Gross Tumor Volume (GTV), as identified on CT and/or MRI, plus a margin (ideally up to 10-mm for chordoma) to allow for microscopic disease extension. Tracking systems, such as Xsight® Spine (Accuray) compare digitally reconstructed radiographs (DRRs) generated from preoperative CT scans with frequently acquired intraoperative radiographs of the spine to identify any 3-D target displacements and global rotations of spinal structures. Translations and global rotations are aligned during patient setup, and automatically corrected during treatment delivery. Critical structures (including spinal cord, cauda equina, nerve roots, intestines, esophagus, trachea, kidneys, and heart) are delineated by the neurosurgeon and the radiation oncologist. An inverse treatment planning algorithm using linear optimization is used to generate a treatment plan with non-isocentric targeting. Plans are created and prescribed individually for each patient based on isodose coverage, dose-volume histograms (DVHs), normal individual tissue doses, and other clinical considerations. Additionally, dose and fractionation prescriptions are shaped by dose volume constraints for the adjacent normal tissues. Treatment margins are added to GTVs based on clinical presentation, histology, and proximity to critical structures. Patients were treated daily, up to 5 days per week. Patients are offered mild sedation, such as benzodiazepines, during the treatment. The use of pre-treatment steroids, anti-emetics, nonsteroidal anti-inflammatory medications, and narcotics are determined according to the individual clinical presentation, proximity of critical structures, and risk of complicating edema.
Recurrence and Survival Outcome
The Georgetown University experience encompassed adult 18 patients. Radiation Dosimetry Depending upon previous irradiation, chordomas were treated on average with a regimen of 5 × 700 cGy to the margin. The isodose lines selected for individual tumors ranged from 60 % to 84 % (median 75 %). Median conformity index was 1.67, median coverage index 90.9 %. The average tumor volume (CTV) was 128 cm3 (range 12.0–457.3 cm3). At a mean follow-up duration of 5.8 years (range, 12 months to 18 years) from diagnosis, 14 patients were alive and 4 patients had died. Patients were followed after CK/SRS for a median of 45.5 months (range, 7–65 months). Overall survival rate by the Kaplan-Meier method was 74.3 % at 5 years after CK/SRS.
The actuarial local control rate was 59.1 % at 5 years. Seven of the eighteen patients (39 %) experienced recurrence at a mean 16 months after CK/SRS (range, 5–38); three of these same patients had recurrences prior to CK/SRS. Four patients were diagnosed with metastases after having undergone previous irradiation (3 CK/SRS, 1 EBRT) to the primary lesion. The disease-specific survival rate was 88.9 % at 5 years. Two of the four deaths were unrelated to chordoma, occurring in the absence of any evidence of disease; one death occurred at 7 months of unknown etiology, but was attributed to the chordoma; and one patient died of pulmonary complications following chordoma recurrence. There has been no evidence of recurrence of spinal chordoma in any patient in whom the tumor was totally resected and tumor site irradiated with 37.5 Gy to the tumor margin.
Quality of Life and Pain: The mean physical component of quality of life improved. Scores for the mental component of quality of life remained stable, regardless of whether the patients had undergone surgery and CK/SRS, or irradiation alone. Overall, the mean PCS and MCS scores of patients improved from 33.7 to 57.1 and from 50.3 to 58.4, respectively, and sustained a durable improvement throughout the period of observation. The mean pain score for patients prior to CK/SRS treatment was 40, which decreased to 3 for patients reaching 6 years of follow-up, reflecting a trend for the VAS to decrease (p = 0.11). Most patients were managed with narcotic analgesics and NSAIDS. There was a tendency for MCS scores to increase following surgery (p = 0.09); however, patients varied considerably in their responses over time.
Complications: There were no disabling (Grade 3 or 4) complications noted in this series. Three patients with previously irradiated clival chordomas (1 PBRT, 1 Gamma Knife, 1 EBRT), exhibited decreased vision or diplopia, but not blindness; two of these also had lower cranial nerve palsies. After surgery but before CK/SRS, two patients were hypoesthetic from lumbar and two from sacral radiculopathy; one developed a neurogenic bladder; and one urinary urgency. One patient (Illustrative Case #2) developed a neurological change after CK/SRS; she had been treated with 37.5 Gy to C3 and C4, and manifested hypoesthesia due to C4 radiculopathy and transient paresthesias possibly due to excessive spinal cord dose, although there were no findings on neurological exam. Two patients who received preoperative CK/SRS developed abdominal infections.
Illustrative Case # 1
A 53-year-old woman presented at another institution with primary complaints of perineal numbness, bladder dysfunction, and pain in her lower back and buttocks, which worsened upon sitting. MRI revealed what initially appeared to be a pseudomeningeocele secondary to a sacral cyst, but sacral laminectomy and biopsy revealed a chordoma. Two weeks later, she undewrwent intralesional resection of the dorsal portion of the chordoma (~30 % tumor volume removed) down to the level of the sacral roots. 2 months after surgery, her symptoms resolved, and she was referred for CK/SRS at GUH (Fig. 31.2a). Treatment was administered in 5 sessions of 750 cGy to the 75 % isodose line around a target volume (CTV) of 457.3 cm3 with 90.5 % tumor coverage. MRI imaging revealed decreased size of the anterior spherical mass of irradiated chordoma from 13.4 to 2.5 cm3 at 3 years of follow-up (Fig. 31.2b). At this point, the patient developed a marginal recurrence at the L5 level; further treatment was recommended, but the patient, who lived in a remote location, unilaterally decided upon hospice.
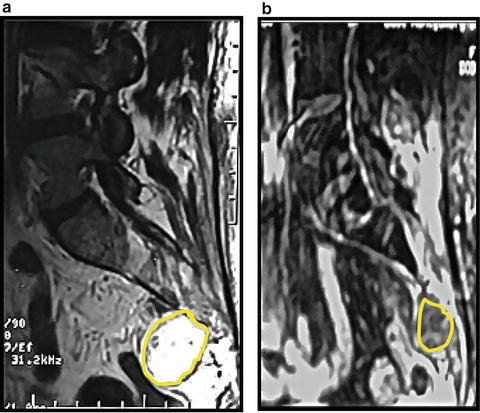

Fig. 31.2
MRI, Sagittal views, of sacrum in a 53 year old woman with sacral chordoma. (a) Patient underwent sacral laminectomy and resection of the dorsal component of the chordoma (approximately 1/3 tumor mass) down the level of the lumbosacral nerve roots. MRI shows the remaining spherical, pre-sacral component of chordomas (arrows) with a volume 13.4 cm3, treated with 37.5 Gy in 5 sessions to the margin. (b) At 3 years, the pre-sacral mass (outlined) has reduced in size to 2.5 cm3 3 years post-CK/SRS
Illustrative Case #2
A 29-year-old woman presented with right-shoulder and arm pain, and MRI revealed a 22.9-cm3 lesion on the left side of C3-4 vertebrae (Fig. 31.3a). Biopsy demonstrated a chordoma. She was referred for CK/SRS and received five fractions of 750 cGy (37.5-Gy total dose) to the 75 % isodose line with a coverage index of 89.04 %. Subsequently, she experienced C4 radiculopathy and mild, transient paresthesias. At 67 months, she had no pain, no neurological deficits, and no evidence of chordoma recurrence by MRI (Fig. 31.3b).

Fig. 31.3
MRI, Axial views, C3 level, T2 weighted images of a 29 year old woman who presented with right shoulder and arm pain; MRI revealed a left C3-4 chordoma (arrows) (a) She received 37.5Gy in five sessions of 750 cGy to 75 % isodose line; (b) At 67 months there is no pain, mild left C4 hypoesthesia, and no evidence of chordoma recurrence
Results of Hypo-Fractionated Irradition
The GUH series shows an actuarial local control rate at 5 years between 50 % and 60 % for the typical fractionation scheme of 700 cGy × 5 fractions = 3,500 cGy. Single-fraction data are available from a Gamma Knife study (Krishnan et al. 2005). In that series of 25 chordomas, the 5-year local control was 32 % with a median dose of 15 Gy to the margin. Martin et al. (2007) reported on a Pittsburgh series of 18 chordomas treated with the Gamma Knife and found a 5-year actuarial local-control rate of 62.9 % for a mean tumor dose of 16 Gy to the margin. Standard fractionated radiation series, where the fraction dose is typically 1.8–2.0 Gy per fraction, have reviewed the published literature on fractionated radiation for chordomas. Their data includes dose, fraction and follow-up sufficient to determine 5-year local control with standard fractionation schemes. Tai et al. (2005). Although their analysis did not observe a dose–response relationship for local control, when the patient data were limited to standard fractionation (1.8–2.1 Gy fractions) and combined with further data from Douglas and Fowler (1976), a dose–response relationship for this fraction size did become evident: doses less than 50 Gy resulted in 5-year local control in 25 % (2/8), dose of 50–60 Gy resulted in 47 % local control (8/17), and doses in excess of 60 Gy resulted in 60 % local control (3/5).
Optimal Radiation Strategy
The rationale for treatment of chordoma should be founded upon the understanding of radiation’s effect on tumor cells. The α/β ratio is a mathematical expression of a particular tissue’s sensitivity to fractionated irradiation, represented in Gray (Gy). α/β ratio represents the fractionation sensitivity to low-dose fractions relative to high-dose fractions. It is not a measure of overall radiation sensitivity, but rather the sensitivity to varying the dose-per-fraction. The response of cancer cells, as well as normal cells, are typically modeled mathematically by a two-parameter model consisting of α and β, with the α component reflecting the contribution of single-hit radiation effects and β representing multiple-hit effects on a cellular target. The effect of radiation is dependent on this linear (α) component of fraction dose and a quadratic (β) component of fraction dose, and therefore radiation sensitivity can be determined by these parameters with the biological effect being proportional to 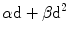 , where d is radiation dose (Douglas and Fowler 1976). The α/β ratio represents the dose where biological effects, such as cell killing, are equally contributed by linear (α) effects and quadratic (β) effects. Tumors with a low α/β ratio (i.e. <4 Gy) will be more sensitive to the effects of a few high-dose fractions; a tumor with a high α/β ratio (i.e. >8 Gy) will be more sensitive to multiple-fractions. Most metastatic cancers are believed to have relatively high α/β ratios, which motivates the current standard radiation therapy treatment protocols that typically use 20–40 smaller dose fractions (Douglas and Fowler 1976). Some tumors, however, have low α/β ratios, such as prostate cancer, which is believed to have an α/β ratio perhaps as low as 1.5 Gy (King and Fowler 2001) and liposarcoma, which appears to have an α/β ratio less than 1 Gy (Thames and Suit 1986). These tumors would be expected to be sensitive to the effects of large fraction doses typical of single-fraction or staged radiosurgery. The α/β ratio of chordoma is unknown, though various ratios have been assigned, based on limited data, ranging from 7 to 10 Gy (Tai et al. 1995; Gwak et al. 2006). We determined this ratio for chordomas using the current series data with other published series of chordoma irradiation. Although relatively elegant in concept, determination of the α/β ratio for tumors is fraught with statistical uncertainties. Typically, this ratio is determined by a Fractionation Equivalent (FE) plot. To use this plot, the dose–response relationship is used:
, where d is radiation dose (Douglas and Fowler 1976). The α/β ratio represents the dose where biological effects, such as cell killing, are equally contributed by linear (α) effects and quadratic (β) effects. Tumors with a low α/β ratio (i.e. <4 Gy) will be more sensitive to the effects of a few high-dose fractions; a tumor with a high α/β ratio (i.e. >8 Gy) will be more sensitive to multiple-fractions. Most metastatic cancers are believed to have relatively high α/β ratios, which motivates the current standard radiation therapy treatment protocols that typically use 20–40 smaller dose fractions (Douglas and Fowler 1976). Some tumors, however, have low α/β ratios, such as prostate cancer, which is believed to have an α/β ratio perhaps as low as 1.5 Gy (King and Fowler 2001) and liposarcoma, which appears to have an α/β ratio less than 1 Gy (Thames and Suit 1986). These tumors would be expected to be sensitive to the effects of large fraction doses typical of single-fraction or staged radiosurgery. The α/β ratio of chordoma is unknown, though various ratios have been assigned, based on limited data, ranging from 7 to 10 Gy (Tai et al. 1995; Gwak et al. 2006). We determined this ratio for chordomas using the current series data with other published series of chordoma irradiation. Although relatively elegant in concept, determination of the α/β ratio for tumors is fraught with statistical uncertainties. Typically, this ratio is determined by a Fractionation Equivalent (FE) plot. To use this plot, the dose–response relationship is used:
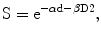 where S is the fraction of cells surviving a radiation dose D. Dividing this dose into n equal fractions gives:
where S is the fraction of cells surviving a radiation dose D. Dividing this dose into n equal fractions gives:
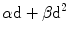 , where d is radiation dose (Douglas and Fowler 1976). The α/β ratio represents the dose where biological effects, such as cell killing, are equally contributed by linear (α) effects and quadratic (β) effects. Tumors with a low α/β ratio (i.e. <4 Gy) will be more sensitive to the effects of a few high-dose fractions; a tumor with a high α/β ratio (i.e. >8 Gy) will be more sensitive to multiple-fractions. Most metastatic cancers are believed to have relatively high α/β ratios, which motivates the current standard radiation therapy treatment protocols that typically use 20–40 smaller dose fractions (Douglas and Fowler 1976). Some tumors, however, have low α/β ratios, such as prostate cancer, which is believed to have an α/β ratio perhaps as low as 1.5 Gy (King and Fowler 2001) and liposarcoma, which appears to have an α/β ratio less than 1 Gy (Thames and Suit 1986). These tumors would be expected to be sensitive to the effects of large fraction doses typical of single-fraction or staged radiosurgery. The α/β ratio of chordoma is unknown, though various ratios have been assigned, based on limited data, ranging from 7 to 10 Gy (Tai et al. 1995; Gwak et al. 2006). We determined this ratio for chordomas using the current series data with other published series of chordoma irradiation. Although relatively elegant in concept, determination of the α/β ratio for tumors is fraught with statistical uncertainties. Typically, this ratio is determined by a Fractionation Equivalent (FE) plot. To use this plot, the dose–response relationship is used:
, where d is radiation dose (Douglas and Fowler 1976). The α/β ratio represents the dose where biological effects, such as cell killing, are equally contributed by linear (α) effects and quadratic (β) effects. Tumors with a low α/β ratio (i.e. <4 Gy) will be more sensitive to the effects of a few high-dose fractions; a tumor with a high α/β ratio (i.e. >8 Gy) will be more sensitive to multiple-fractions. Most metastatic cancers are believed to have relatively high α/β ratios, which motivates the current standard radiation therapy treatment protocols that typically use 20–40 smaller dose fractions (Douglas and Fowler 1976). Some tumors, however, have low α/β ratios, such as prostate cancer, which is believed to have an α/β ratio perhaps as low as 1.5 Gy (King and Fowler 2001) and liposarcoma, which appears to have an α/β ratio less than 1 Gy (Thames and Suit 1986). These tumors would be expected to be sensitive to the effects of large fraction doses typical of single-fraction or staged radiosurgery. The α/β ratio of chordoma is unknown, though various ratios have been assigned, based on limited data, ranging from 7 to 10 Gy (Tai et al. 1995; Gwak et al. 2006). We determined this ratio for chordomas using the current series data with other published series of chordoma irradiation. Although relatively elegant in concept, determination of the α/β ratio for tumors is fraught with statistical uncertainties. Typically, this ratio is determined by a Fractionation Equivalent (FE) plot. To use this plot, the dose–response relationship is used: