A. Basic features (all of which need to be met)
1. The increase in symptom severity was experiences on five out of seven days during the previous week
2. The increase in symptom severity is not accounted for by other factors such as a change in medical status, lifestyle, or the natural progression of the disorder
3. It is assumed that there has been a prior positive response to treatment
In addition, either B or C or both have to be met
B. Persisting (although not immediate) paradoxical response to treatment: RLS symptom severity increases some time after a dose increase, and improves some time after a dose decrease
C. Earlier onset of symptoms
1. An earlier onset by at least 4 h
OR
2. An earlier onset (between 2 and 4 h) occurs with one of the following compared to symptom status before treatment
3. (A) Shorter latency to symptoms when at rest
4. (B) Spreading of symptoms to other body parts
5. (C) Intensity of symptoms is greater [or increase in periodic limb movements (PLM) if measured by polysomnography (PSG) or the suggested immobilization test (SIT); suggested immobilization test (SIT)]
6. (D) Duration of relief from treatment is shorter
Other triggers include alcohol, dietary products, inadequate sleep, stress, comorbid diseases, lifestyle changes, and exercise. Despite the only study that evaluated the relationship between RLS and alcohol [45] having found an epidemiological association between RLS and low alcohol consumption, many patients report worsening of their RLS shortly after drinking alcoholic beverages. This study also found a link between lack of exercise and RLS. Moderate exercise has been demonstrated in one study to improve RLS symptoms with a 12-week conditioning program of aerobic and lower body resistance training 3 days per week [46]. However, there is anecdotal evidence that vigorous exercise may exacerbate RLS, sometimes even markedly. There is also anecdotal evidence for anxiety and stress worsening RLS, and the epidemiological study above [45] found a relationship between RLS and poor mental health status. Comorbid disease may result in worsening RLS symptoms. In clinical practice, patients with pain or discomfort from conditions such as back pain or neuropathy find that their RLS has worsened. This relationship may not be immediately apparent and may require extensive questioning to determine the temporal relationship between the comorbid condition and the exacerbation of RLS symptoms. Patients often report worsening of RLS symptoms after surgical procedures or trauma even well after full healing has occurred. There are no studies to support any foods worsening RLS but patients often report that refined carbohydrates, gluten, and ice cream may exacerbate their symptoms. Instituting proper sleep hygiene has been recommended for RLS patients by a few review articles [47–49], but there are no studies to support this advice. However, since it is thought that RLS symptoms increase with sleepiness, inadequate sleep may be a significant factor that could worsen RLS symptoms. Increases in RLS symptoms may also occur with lifestyle changes that result in decreased activity such as retirement or changing to a deskbound job. Patients may not realize that the increased sedentary time may be the cause of their increased RLS symptoms.
Low iron levels have been associated with RLS and treating with oral iron has been found to improve RLS symptoms [50, 51]. Any RLS patient who experiences worsening of RLS symptoms should have a serum ferritin level and if below 50–75 mcg/l, further blood testing should be performed (serum iron, iron binding capacity, and CBC) and treatment with iron therapy should be considered. Additionally, underlying etiologies of decreased iron such as gastrointestinal bleeding or cancer should be investigated although many RLS patients have low iron levels that cannot be explained.
Tolerance
Drug tolerance is defined as a diminution in the response to a drug after prolonged exposure requiring an increased dose to produce the previous response. Tolerance to DAs is thought to be an early stage of augmentation (discussed below) and has been documented in the medical literature [52] and is seen often clinically. It usually develops after several months or years of drug usage.
Once all the above triggers that might worsen RLS have been ruled out, tolerance may be difficult to differentiate from progression of disease and augmentation (which is discussed below). Since tolerance is reversible, stopping the drug for a short time (typically a few weeks or more) will reestablish the effectiveness of the drug which does not occur with disease progression.
There are several actions available when tolerance to DAs occurs. If the DA dose is relatively low, its dose may be increased to the upper limits defined above. If further tolerance develops, care should be taken with further increases of the drug to avoid the emergence of augmentation. Another DA may be substituted but it is quite likely that cross-tolerance may develop. If that occurs, then treatment with α2δ drug and/or opioids as discussed above may be instituted. For patients who prefer the DAs to those other treatments, rotation drug therapy may be helpful. The α2δ drug and/or opioid can be taken for a few weeks then the DA resumed for weeks or months until significant tolerance develops again.
Intolerable Side Effects
Early Treatment Adverse Reactions
The short-acting DAs tend to share similar side effects. The most common one that occurs with the initiation of therapy is nausea. This typically manifests about 1–3 h after the medications are taken and corresponds to the peak of dopamine agonist blood levels. The nausea may be self-limiting and diminish with time but some patients have persistent severe symptoms that lead to discontinuation of the drug. There are several strategies for dealing with the nausea. The medication can be taken with food which often blunts or eliminates this issue but results in about an hour delay of the onset of action. Anti-nausea medications may prevent the nausea but most of these make RLS worse. The “RLS friendly” anti-nausea drugs (Table 13.1) may be taken prior to the DAs but adding a drug on a regular basis should only be considered when other treatment options are not available. Switching from one DA to another may reduce or eliminate the nausea.
Other common early treatment-emergent side effects include fatigue, sleepiness, dizziness, insomnia, nasopharyngitis, and headache. These early problems may resolve with time but if they are severe enough and do not resolve then the DA should likely be stopped. As with nausea, changing to another DA may be helpful. Otherwise, the patient should be changed to a different class of RLS drugs (α2δ drugs or opioids).
Late Treatment Adverse Reactions
The most common late treatment-emergent side effect other than augmentation is ICD (Impulse Control Disorders), which occurs in 6–17% of RLS patients taking dopamine agonists [53]. The ICDs constitute a group of psychiatric disorders in DSM-IV, their essential feature being a failure to resist an impulse, drive, or temptation to perform an act that is harmful to the person or to others [54]. The behaviors that are typically manifested include pathologic gambling, hypersexuality, compulsive shopping, compulsive eating, compulsive medication use and punding. It is often difficult to diagnose the ICD in its early stages as patients are generally very secretive about their abnormal behavior and will not acknowledge their problem until it is exposed by family members or close friends. Treatments of the ICD include changing to another dopamine agonist, reducing the dose of the dopamine or eliminating dopamine agonists completely. When the dopamine agonist dose is reduced or eliminated, other classes of medication (α2δ drugs or opioids) typically need to be added or substituted.
Augmentation
After Akpinar described the use of levodopa for treating RLS in 1982 [1], this drug was widely prescribed and dramatically helped many RLS sufferers. However, after an initial period of relief, most patients developed very increased RLS symptoms that required higher and higher doses to obtain a therapeutic response, symptoms started occurring earlier in the day and expanded to the arms and other body parts. This phenomenon was quite puzzling until the term augmentation was first described in 1996 [2]. Augmentation was further defined with the development of criteria for its diagnosis by an NIH workshop in 2003 [55] (Fig. 13.1) [56] and subsequently by a consensus conference at the Max Planck Institute (called the MPI criteria) in 2007 [57] (Table 13.1). When it became apparent that the majority of patients taking levodopa for RLS were developing augmentation [2], physicians switched the treatment to a dopamine agonist. Pergolide was one of the first DAs used to treat RLS. It worked quite well to treat the RLS symptoms of those augmented patients but its use was often limited by its side effects. Therefore, most physicians treating RLS with or without augmentation switched to ropinirole and pramipexole when they became available in 1997 for Parkinson’s disease and especially after they were FDA approved for RLS in 2005 and 2006 respectively. The majority of these patients seemed to do very well with short-acting DA treatment and there appeared to be only minor concerns about augmentation. However, more recent studies using the more current NIH or MPI criteria for diagnosing RLS have found much higher rates of augmentation; up to 8% per year in a community sample study [55] and 5–7% per year in 10-year long retrospective study [33]. Augmentation symptoms from DAs tends to be severe often resulting in discontinuation of the DA [57]. Since the augmentation problem may develop at any time during 10 or more years of treatment, it is not often identified as such by most primary care physicians or even many specialists who are the local consultants for difficult RLS cases. These patients are thought to have gradual progression of their disease and are most often treated with escalating doses of DAs, frequently even into the high Parkinson’s disease ranges. This has resulted in patients with augmentation making up the majority of RLS referrals to doctors, who have greater expertise with RLS such as movement disorder specialists [58].
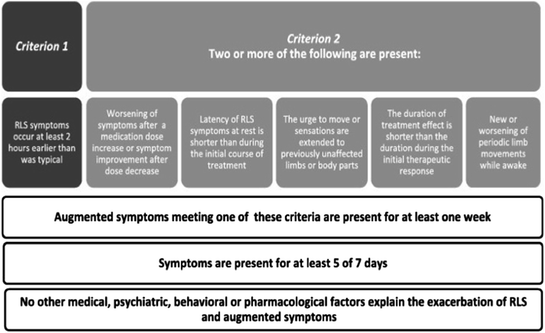

Fig. 13.1
NIH diagnostic criteria for diagnosing RLS augmentation
As discussed above, one of the most important aspects of treating the augmentation caused by short-acting DAs is the recognition of the problem. Although the diagnostic criteria for augmentation are well defined [54, 56], there is considerable overlap of its features with other conditions that may confuse the diagnosis. The most common misdiagnosis of augmentation is the natural progression of the disease. This misunderstanding may have been further propagated by earlier studies, which found that augmentation occurred no later than 4 months [59] or 2.5 years [60] after the initiation of short-acting DA therapy. It is now clear that augmentation can occur for up to 10 years [57] or longer. When the augmentation symptoms onset within a few months of treatment, the diagnosis is much more obvious (patients will typically complain about the relative sudden worsening after the short period of symptom relief). However, as discussed above, when augmentation arises after several years of treatment, most physicians will assume it is due to progression of the disease. The only definite way to differentiate between these two issues is to stop the short-acting DA. Patients with augmentation will show improvement of their symptoms within a few weeks or months (after an initial period of marked worsening) while those with natural progression of the disease will not. However, since the cessation of treatment in augmentation cases causes a temporary dramatic increase of RLS symptoms resulting in significant suffering, this is usually not a very practical approach. Therefore, physicians should be very suspicious of augmentation whenever the dose of a short-acting DA must be increased. With augmentation, there will be further dose increases after shorter and shorter intervals while natural progression requires dose increases over prolonged intervals (personal observation).
Before augmentation is diagnosed, triggers that may impact RLS should be ruled out [56]. As discussed above, a thorough review of the patient’s medications, lifestyle, and iron status should be performed. Removing the trigger may improve the RLS and obviate the need for additional RLS medication or concerns of augmentation. Rebound should not be confused with augmentation. Since rebound is an end of the dose effect of a short-acting DA, symptoms will occur as the drug’s action is waning which typically occurs about 6–8 h after the medication is taken. Therefore, the clinical presentation of rebound is a worsening of RLS symptoms several hours after taking the DA rather than a few hours prior to taking the DA as with augmentation. Tolerance to the DAs also shares features of augmentation and may even be an early stage or subtype of augmentation [52]. However, despite tolerance producing a decreased effectiveness of a given DA dose with time that requires higher doses of that DA, it does not cause an earlier onset of symptoms or expansion of symptoms to other body parts.
Treatment of Augmentation from Short-Acting Dopamine Agonists
Prevention
Since the pathophysiology of augmentation is not fully understood, schemes to prevent it must rely on observational data. Increased augmentation rates have been associated with lower serum ferritin levels [61, 62]. However, there are no guidelines for the ideal serum ferritin levels that would prevent augmentation. Until further studies are performed, it would seem reasonable to suggest that the previous ferritin goal of >50–75 mcg/l [50, 51] discussed above (in the Triggers section) is a suitable target range that might forestall the development of augmentation for patients on short-acting DAs. Keeping the dose of the DA as low as possible has also been recommended to prevent augmentation as higher doses have been thought to be associated with increased risk of augmentation [14, 52, 56, 63, 64]. As such, recommendations to limit the daily dose of ropinirole to as low as 1 mg and pramipexole to 0.25 mg have been suggested to decrease the development of augmentation [11, 65]. However, it should be noted that augmentation may occur at even the lowest available doses of these short-acting DAs. In fact, one article [66] has gone so far as to suggest that perhaps dopaminergics should not be used at all to treat RLS due to the augmentation issue. The authors of this article use rotation therapy (alternating a short-acting DA with clonazepam and low-potency opioids) to avoid the sustained use of a DA which they believe may heighten the risk of augmentation. It has been suggested that the risk of augmentation is inversely related to half-life of the DA [4, 67, 68]. Although this phenomenon has not been proven, it appears that augmentation rates are lower with the long-acting cabergoline (5.6%) [69] and the rotigotine patch (5% with the 1–3 mg/day FDA approved doses) [70]. Therefore, it may be prudent to consider using a long-acting DA instead of a short-acting one when this class of medication is being considered for treating RLS. However, issues with fibrotic heart valve formation should limit the use of cabergoline [71]. One of the concerns with the decreased augmentation seen with long-acting DAs is that their long duration of action may obscure the diagnosis of augmentation by treating the earlier onset of RLS symptoms, which is one of the cardinal symptoms for identifying augmentation.
Management of Augmentation
So far there are only a few articles [4, 12, 15, 56, 58, 64, 65, 72] in the medical literature that discuss the treatment of augmentation. One of the articles published in 2007 [70], is authored by several experts in the field and is still quite clinically relevant. This article contains the first and only algorithm written so far on how to manage augmentation (Fig. 13.2). This algorithm is discussed below with further suggestions based on additional knowledge and drugs that that have become available since the algorithm’s creation in 2007.


Fig. 13.2
RLS augmentation treatment algorithm
When augmentation is suspected, physicians should search for possible triggers that may have worsened the RLS symptoms (see Triggers above). A serum ferritin level should be checked and patients should be questioned about drug and lifestyle changes that may affect their RLS symptoms [12, 15].
Management of RLS that Is Not Clinically Significant
Augmentation symptoms may range from minimal and barely bothersome to extremely severe and disruptive. Physicians have to decide at what point in the continuum that they must intervene. Mild augmentation with symptoms that are not disruptive may not need any action. Based on the MPI Consensus Conference [56], the algorithm suggests that augmentation does not need to be treated until the symptoms become clinically significant as defined below:
Any One of More of a–e Below
- a.
Change in daily activities and/or behavior (e.g., the patient stops riding in cars in the afternoon) due to augmentation.
- b.
Negative impact on the patient’s quality of life (sleep, mood, etc.) due to augmentation.
- c.
Need to change the treatment dose or the patient needs to take the dose earlier in the day (e.g., dividing the dose).
- d.
Adjustments in concomitant medication are made to compensate for augmented RLS symptoms (e.g., an increased intake of analgesics or hypnotics to cover an increase in symptom intensity).
- e.
Any other aspect as judged by the evaluator (should be specified).
Patients without clinically significant symptoms can simply be watched closely for any further development of augmentation symptoms without altering their therapy. The percentage of patients with this clinically insignificant augmentation that progress to more severe augmentation is unknown. However, based upon clinical experience, these patients are clearly at higher risk of developing worsening augmentation problems over the next several years. Since it is much easier to modify therapy earlier in the augmentation process when symptoms are less severe, this may be an opportune time to intervene and change treatment. Although the augmentation algorithm (Fig. 13.2) indicates that no treatment adjustments are necessary for these milder augmented patients, several treatment options are presented below.
Options for Changing Current Short-Acting DA Therapy
Changing to Another Short-Acting DA
There have been some suggestions that augmentation that has developed with one short-acting dopamine may not occur when substituting another [4, 64]. However, there is really no evidence in the medical literature that substantiates this supposition and based upon clinical experience many experts believe that it is better to reduce or eliminate all short-acting DAs [70] when deciding to treat augmented patients. Nevertheless, if no other options exist, it may be worth trying this substitution.
Reducing the Dose of the Short-Acting DA
There is no clinical research that validates the approach to decrease dose of the short-acting DA to treat augmentation but physicians have used this technique with some success. This approach may also be easier with milder cases of augmentation as the temporary increase in RLS symptom intensity and duration upon reducing the DA dose is significantly less than with severely augmented patients. There are no clear guidelines on how much to decrease the dose but most physicians reduce the current dose by at least 50% with a possible goal of reaching the lowest available dose. Leaving the patient with some DA medication may prevent the more intense and prolonged dopamine withdrawal increase in RLS symptoms when completely eliminating the DA. Some patients may adjust to lower levels of short-acting DA therapy with a very significant decrease in RLS symptoms/augmentation and need no additional therapy. However, many of these patients will require additional therapy (but perhaps less than those who completely eliminate the DA) with either an α2δ drug or an opioid. It is not known whether leaving a low dose of the DA that caused the augmentation may result in a reemergence of the augmentation with time so these patients should be watched closely for any signs of this problem.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




