CHAPTER 33 TREMOR
Tremor is the most common movement disorder encountered in clinical neurology. It denotes a rhythmic involuntary movement of one or several regions of the body.1 Although most tremors are pathological, a low-amplitude physiological action tremor can also be detected in healthy subjects and may even be of functional relevance for normal motor control.2 Pathological tremor is visible to the naked eye and mostly interferes with normal motor function. The disabilities caused by these tremors are as diverse as their clinical appearance, pathophysiology, and etiologies. Although there are numerous medical treatment options, their efficacy is limited, and therefore refined stereotactic surgical approaches have become increasingly important. Here, we provide some general clinical definitions and then describe all these aspects for each of the most important pathological tremor syndromes separately.
CLINICAL DEFINITIONS
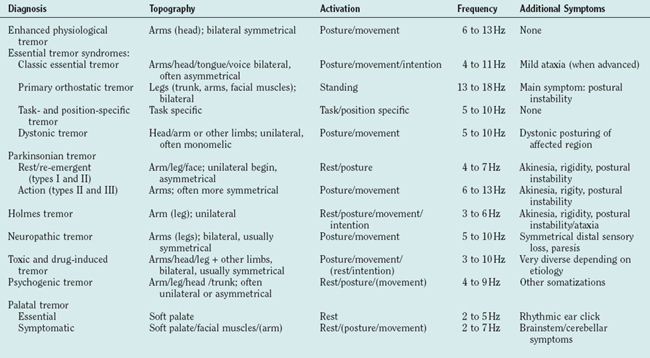
The clinical examination of tremor patients should focus on certain aspects of the tremor that form the basis for the differential diagnosis (Tables 33-1 and 33-2) and should always be documented:
ENHANCED PHYSIOLOGICAL TREMOR
Normal physiological tremor is an action tremor and usually not visible. It can only be measured with sensitive accelerometers. An increase of the amplitude leads to enhanced physiological tremor (EPT). The pathological tremor amplitudes are typically short lived and reversible once the cause (see Etiology) is removed. Other neurological symptoms or diseases that could cause tremor must be excluded.1
Epidemiology
There are no studies available on the epidemiology of EPT as a whole. The short-lived transient form is very likely the most common form of tremor; most people have experienced this on stressful or frightening occasions. Some of the causes (side effect of drugs, endogenous intoxication, etc.) of EPT are common, so it is likely one of the most common forms of tremor.
Pathophysiology
EPT relies on the same physiological mechanism as normal physiological tremor, and the physiology of normal tremor is meanwhile well defined. Two different physiological tremor mechanisms have been established. The first is based on simple mechanical properties. Any movable limb can be regarded as a pendulum with the capability to swing rhythmically, that is to oscillate. These oscillations automatically assume the resonant frequency of this limb, which is dependent on its mechanical properties—the greater its weight, the lower is its resonant frequency, and the greater the joint stiffness, the higher is this frequency. Any mechanical perturbation can activate such an oscillation. In the case of the hands, which are most often affected by tremors, the main and most direct mechanical influence comes from the forearm muscles. Indeed, it has been shown that the tremor measured in normal subjects during muscle activation mainly emerges from an amplification of the muscles’ effect on the hand at its resonant frequency.3–5 Thus, although the muscles show normal nonrhythmic isometric activity, they contribute to these resonant oscillations, which account for most of the tremor seen in the physiological situation.6 Such a pure resonant phenomenon does not produce pathological tremors as its amplitude is typically quite low. However, as this low-amplitude oscillation leads to rhythmic activation of muscle receptors, it activates segmental (spinal) or long (e.g., transcortical) reflex loops that can greatly enhance this oscillation. Such a reflex enhancement of the physiological mechanical oscillation is one well-established pathophysiological basis for the emergence of pathological tremor amplitudes in the case of EPT.7
The second less frequent mechanism in physiological tremor is a transmission of oscillatory activity within the central nervous system to the peripheral muscles. The rhythmic activity of the muscles then leads to tremor. In contrast to the mechanical-reflex oscillations, central oscillations occur at the centrally determined frequency and are independent of the limbs’ mechanics.8,9 It has been shown that such a central tremor component is present in a small proportion of normal subjects in parallel to the more common mechanical reflex oscillations.6,10–12 An enhancement of this component is another basis for EPT.13 Such central oscillations generally are the most common pathophysiological mechanism in pathological tremors4,9,14 (see later). Accelerometric tremor recordings with different weight loads in combination with the electromyogram recorded from the driving muscles can distinguish between such central and peripheral resonant (possibly reflex enhanced) tremors.
Etiology
The causes of an enhancement of one or both of the physiological tremor components are diverse. The well-known trembling with excitement, fear, or anxiety is the most common form of EPT. It is believed to be mediated through an increased sympathetic tone that results in a β-adrenergically driven sensitization of the muscle spindles increasing the gain in the reflex loops.7 A similar origin via the sympathetic nervous system has been proposed for the tremor in reflex sympathetic dystrophy.15 The majority of other causes for EPT are related to drugs or toxins that can enhance the peripheral and the central component of physiological tremor (see Toxic Tremors).
Differential Diagnosis
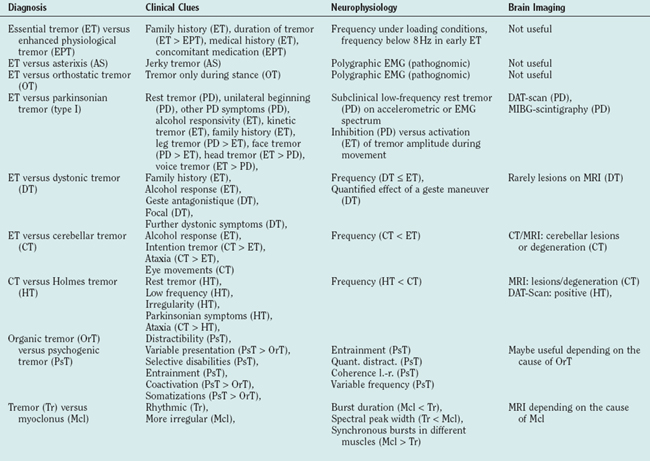
As both EPT and early essential tremor are not accompanied by any other neurological symptoms, they can be difficult to distinguish. The positive family history in essential tremor, its chronic course, and the lack of an overt cause for the tremor are important hints. Sometimes the diagnosis can only be made after having observed the tremor for some time. EPT is usually bilateral and thus any tremor manifesting unilaterally, even with a high frequency and a pure postural component, must be suspected of being a symptomatic tremor (see Table 33-2). Electrophysiology (spectral analysis of accelerometry and electromyography) can be helpful in cases where EPT emerges from a reflex enhancement of physiological tremor, as essential tremor is a centrally driven tremor.14,16 Electromyographic bursts below 8 Hz seem to be in favor of essential tremor rather than EPT.17
ESSENTIAL TREMOR
Essential tremor is a slowly progressive tremor disorder that causes severe disability but is not life limiting. It is defined by the following core criteria:1,18
 Bilateral tremor of the hands or forearms with predominant kinetic tremor and resting tremor only in advanced stages of the disease
Bilateral tremor of the hands or forearms with predominant kinetic tremor and resting tremor only in advanced stages of the disease And absence of other neurological signs with the exception of cogwheel phenomenon and slight gait disturbances
And absence of other neurological signs with the exception of cogwheel phenomenon and slight gait disturbancesSome criteria involve the severity of the tremor,19 which are falling short when applied to a slowly developing condition. But the criteria mentioned here also have difficulties as the differential diagnosis to enhanced physiological tremor is not yet clear enough.
Essential tremor usually starts with a postural tremor but can still be suppressed during goal-directed movements. In advanced stages an intention tremor can develop. This has been found in roughly 50% of an outpatient population and is accompanied by signs of cerebellar dysfunction of hand movements like movement overshoot and slowness of movements.20 In more advanced stages a tremor at rest can develop. Also, a mild gait disorder prominent during tandem gait is frequently found.21 Oculomotor disturbances are found with subtle electrophysiological techniques but cannot be detected by means of clinical assessment. The condition may begin very early in life and the incidence is increasing above 40 years with a mean onset of 35 to 45 years in different studies and an almost complete penetrance at the age of 60.22,23 The topographic distribution shows hand tremor in 94%, head tremor in 33%, voice tremor in 16%, jaw tremor in 8%, facial tremor in 3%, leg tremor in 12%, and tremor of the trunk in 3% of the patients.22–24 In some of the topographic regions (e.g., head, voice, and chin), tremor may occur in isolation.25 About 50% to 90% of the patients improve with ingestion of alcohol, which can be used as an important feature of medical history.
So far only few data are available on the progression of the condition and have shown a decrease of tremor frequency and a tendency to develop larger amplitudes.26 Intention tremor develops at various intervals between 3 and 30 years after the onset of postural tremor.20 The disease-related disability varies significantly and is dependent on the severity of intention tremor.27 For a generic quality of life questionnaire (SF-36), essential tremor patients scored worse in all eight SF-36 domains. Tremor severity correlated with some of the physical domains as well as with social function of the mental domains.28 An essential tremor-specific quality of life questionnaire has been validated.29 Up to 25% of the patients seeking medical attention must change jobs or retire from work.30
Nontremor symptoms have been described as a mild frontal dysexecutive syndrome31 and slight personality changes,28,32 which have both been interpreted to reflect a cerebellar dysfunction. Furthermore, a deficit of hearing33 and olfaction was found independent from disease duration and severity.34
Epidemiology
The prevalence of essential tremor has been assessed in many studies to be between 1.3% and 5.1% when only studies with convincing methodological approaches are taken into account.35 Such methodological problems are the lack of a test for the validation of the diagnosis as many other conditions may manifest with a slowly progressive action tremor. Convincing studies on the incidence are not available. Patients with essential tremor were found in retrospective studies to live longer than those without essential tremor,36 but other studies failed to find this. There is at least no evidence for a shortening of life span due to essential tremor.
Etiology
The majority of cases are hereditary. The families that have been described hitherto have shown an autosomal dominant inheritance with an almost complete penetrance at the age of 60 years. Twin studies allow to estimate the heritability, which was estimated to be low in a relatively small study37 but almost 100% in a larger study of twins of old age (>70 years).38 Thus, in families with familial essential tremor, the heritability seems to be extremely high and the role of environmental factors is probably limited. However, there is a proportion of approximately 20% to 40% of the patients who have no family history of essential tremor and therefore may not be genetic. Linkage has been found for two chromosomes, 3q1339 and 2p22.40 For the latter locus, a rare variant of the HS1-BP3 protein has been described,41 which binds to motor neurons and Purkinje cells and regulates the Ca2+/calmodulin-dependent protein kinase activation of tyrosine and tryptophan hydroxylase. Further confirmation is needed. On chromosome 3, a gain of function-mutation of the DRD3-gen is considered to be responsible. The environmental factors that may cause tremor are also understudied. β-Carboline alkaloids are known to cause tremor in animals and humans42 and were found to be elevated in the blood of patients with essential tremor.43 Also, the lead concentration was found to be elevated in essential tremor.44 Controlled epidemiological studies are necessary.
Pathophysiology
The pathophysiology of essential tremor was reviewed45,46 and is covered only briefly here. Essential tremor is likely enhanced by peripheral reflex mechanisms, but its main origin must be within the central nervous system for various reasons: either a preformed mechanism in the brain that is producing rhythmic movements is overactive or pathology has created a system to oscillate, which is usually stable. The oscillator is most likely located within the olivocerebellorubral triangle. The cerebellum shows some mild to moderate signs of malfunction demonstrated in a number of motor tests. The rhythmic movement is obviously mediated through different channels of both hemispheres for the different extremities as the trembling is independent in the four extremities.47 It is assumed that the rhythmic discharges are mediated through the thalamus to the premotor and motor cortex projecting down to the motor neurons. At both locations, tremor-related activity can be detected with electrophysiological techniques. Alternative pathways, mainly from the cerebellar nuclei through reticulospinal pathways to the spinal cord, have been proposed.
Differential Diagnosis
The following criteria are considered red flags for the diagnosis (see also Table 33-2):
 Presence of known causes of enhanced physiological tremor (e.g., drugs, anxiety, depression, hyperthyroidism)
Presence of known causes of enhanced physiological tremor (e.g., drugs, anxiety, depression, hyperthyroidism)Treatment
Tremor of the Hands
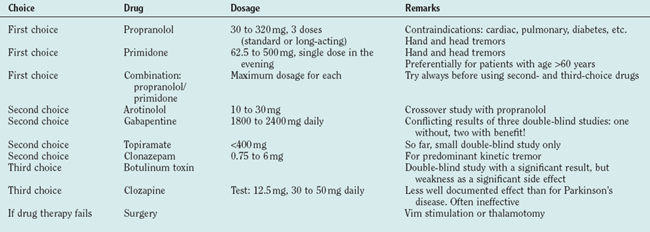
Propranolol and primidone are the drugs of first choice for this indication, and both have been carefully studied (for review, see Findley48). Propranolol was introduced in 197149 as a treatment for essential tremor. Drugs with predominant β1 effects have been shown to be less effective than those acting on the β2 receptor, and none has proved superior to propranolol. Only 25% maintain their initial good response for 2 years. Contraindications are cardiac insufficiency or arrhythmia and diabetes. As propranolol acts on the peripheral (reflex) enhancement of tremors, it is helpful for many other tremors like parkinsonian or cerebellar tremor.50,51 Primidone is efficient for essential tremor52 but tachyphylaxia may occur. The major problems are early adverse effects with nausea, dizziness, sedation, and headache. The combination of propranolol and prinidone is recommended whenever one of the drugs is insufficient. Arotinolol has been tested in a crossover study with a similar effect as propranolol.53 Gabapentin is also effective following two double-blind studies,54 but another double-blind study showed no convincing effect.55 Topiramate was shown to be effective in a small double-blind study.56 Levetiracetam is just beginning to be explored for the treatment of essential tremor and single-dose studies are promising.57,58 Acetazolamide (and methazolamide) are not significantly better than placebo.59 Alprazolam is helpful in essential tremor.60 Clonazepam is recommended for patients with predominant action and intention tremor in essential tremor61 but not effective in uncomplicated essential tremor.62 Botulinum toxin at a dosage of 50 units or 100 U of Botox has a significant but clinically limited effect and carries a high risk of a clinically meaningful but completely reversible paresis63 (Table 33-3 and, for drug dosages, Table 33-4).
Surgery is the accepted treatment for patients resistant to medical treatment and severe disability. Multicenter studies have shown that thalamic deep-brain stimulation is effective,64–67 and one study has shown that deep-brain stimulation of the Vim has a better effect than Vim-thermocoagulation and even fewer side effects.68 The selection of patients for surgery is a crucial point for a good therapeutic effect. Each patient should test the treatments of first choice before surgery and each patient proposed for surgery must have a significant handicap. Gamma knife surgery for the treatment of tremors is proposed in some centers, but prospective studies are lacking and the risks are not yet fully clear.69–71
Head and Voice
Pharmacological treatment of essential head and voice tremor is less efficient than the one of hand tremor. Propranolol and primidone, each alone or both combined, have been recommended72,73 for essential head tremor. Clonazepam is often recommended for this indication, but careful studies are not available. One of the promising therapies for head tremor is the local injection of botulinum toxin.74 Deep brain stimulation is also effective for head and voice tremor. As bilateral thalamotomies carry a high risk of dysarthria and bilateral interventions show better effects on these “midline” tremors,75,76 mostly Vim stimulation is applied.66,77 An evidence-based medicine-based review on treatments for essential tremor was published.78
ORTHOSTATIC TREMOR
Primary orthostatic tremor is a unique tremor syndrome79,80 characterized by a subjective feeling of unsteadiness during stance but only in severe cases during gait. Some patients show sudden falls. None of the patients has problems when sitting and lying. The only clinical finding is sometimes visible but mostly only palpable fine-amplitude rippling of leg muscles. This tremor is suspected mainly based on the complaints of the patients rather than on clinical findings.
Epidemiology
Orthostatic tremor is a relatively rare condition (only small case series have been published adding up to fewer than 200 cases), but epidemiological data are lacking. Other movement disorders are common in orthostatic tremor. The condition occurs only in patients older than 40 years, and in the series of Gerschlager and colleagues,81 the mean age at onset was lower for women (50 years) compared with men (60 years). So far, it is not considered a hereditary disease.
Etiology and Pathophysiology
Orthostatic tremor is considered an idiopathic condition. However, other movement disorders often occur simultaneously: Parkinson’s disease, vascular parkinsonism, and Restless legs syndromes (RLS) have all been described in orthostatic tremor, but there is no convincing evidence that any of these conditions are pathophysiologically related. It is of special interest that dopaminergic terminals are significantly reduced in this condition82 but clinical trials with L-dopa and dopamine agonists are usually unsuccessful.
Surface electromyography (e.g., from the quadriceps femoris muscle) while standing shows a typical (pathognomic) 13- to 18-Hz burst pattern. All of the leg, trunk, and arm muscles show this pattern, which is in many cases absent during tonic innervation when sitting and lying.83–85 Besides asterixis, orthostatic tremor is the only tremulous condition for which electromyography is mandatory for the diagnosis. Arm tremor may occur in roughly one half of the patients and is usually more evident during stance.86,87 The high-frequency electromyographic pattern is coherent in all the muscles of the body,88 leading to the hypothesis that a bilaterally descending system must underlie orthostatic tremor. Such projections originate only from the brainstem and not from the hemispheres. Therefore, the generator for this tremor is assumed to be located within the brainstem or cerebellum.89
Treatment
Orthostatic tremor has been documented to be responsive to clonazepam and primidone.90 Valproate and propranolol were applied in single cases with varying success. Abnormalities of dopaminergic innervation of the striatum have been described, although L-dopa has not consistently shown efficiency.82,91 According to small double-blind studies92,93 and our experience, gabapentin seems to have an excellent and most consistent beneficial effect.94 We use it as the drug of first choice for orthostatic tremor (1800 to 2400 mg daily). The drug of second choice, in our hands, is clonazepam.
PARKINSONIAN TREMORS
Parkinsonian tremor has been defined as tremor that occurs in Parkinson’s disease.1 The most common forms are the following:
Classic parkinsonian tremor (type I) is defined as tremor at rest (ideally resting on a couch) that increases in amplitude under mental stress and is suppressed during initiation of a movement and often during the course of a movement. Tremor frequency is 4 to 6 Hz but can be as high as 6 Hz, especially in early Parkinson’s disease. It may also be seen in the hands during walking or when sitting as a typical pill-rolling tremor of the hand. The postural/kinetic tremor (with similar frequencies for rest and postural/kinetic tremors) seems to be a continuation of the resting tremor under postural and action conditions. The frequencies for resting and postural/action tremor can be considered to be equal if they do not differ by more than 1.5 Hz. Unilateral tremor or leg tremor are often seen and are typical for type I tremor.
A clinically important specific variant of Parkinson’s disease is the monosymptomatic tremor at rest or benign tremulous parkinsonism. This is defined as a classic Parkinson’s disease type I tremor without other symptoms sufficient to diagnose Parkinson’s disease.1
In some patients, a second form of postural and action tremor with a different frequency from resting tremor (>1.5 Hz) may occur, which is labeled type II tremor. This postural/action tremor can be extremely disabling. Some patients have a predominant postural tremor in addition to their resting tremor. The postural/action tremor has a higher and non-harmonically related frequency to the resting tremor. This form is rare (<15% of patients with Parkinson’s disease) and has often been described as a combination of an essential tremor with Parkinson’s disease.95 Some of these patients had their postural tremor long before the onset of other symptoms of Parkinson’s disease. A high-frequency action tremor also described as “rippling” is often found in Parkinson’s disease and has been described as type III tremor in Parkinson’s disease.1
Etiology and Pathophysiology
It is one of the mysteries of Parkinson’s disease that the typical type I tremor is a symptom with such a high specificity for Parkinson’s disease but that the symptom of tremor does not correlate with disease progression96,97 nor does tremor severity correlate with the amount of dopaminergic degeneration measured with positron emission tomography or single-photon emission computed tomography imaging.98–100 Pathology suggests that in patients with predominant tremor the retrorubral Aδ part of the substantia nigra is specifically degenerating101–103 but that there are no clear-cut differences in the positron emission tomography imaging of the presynaptic or postsynaptic dopaminergic terminals in patients with monosymptomatic tremor at rest compared with classic Parkinson’s disease patients.104,105 Interestingly, reduction in 5-hydroxytryptamine1A binding in the midbrain raphe region is correlating with tremor severity but not with rigidity or bradykinesia.106 Thus, degeneration of transmitter systems other than dopamine may be responsible for the erratic behavior of tremor as a symptom in Parkinson’s disease. Nevertheless, L-dopa and dopamine agonists are potent drugs to treat Parkinson’s disease tremor.
Beyond all of these unsolved problems, animal experiments and human data converge to suggest that parkinsonian tremor is generated within the basal ganglia.107 In the 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) model of Parkinson’s disease it has been shown that the cells within the basal ganglia loop are topographically organized through the whole loop and well segregated for the different muscle groups and functional regions. In MPTP animals, these cells become abnormally synchronized, and this may be the reason for synchronized activity leading to peripheral tremor.108 Recordings in humans are compatible with this view.109
Treatment
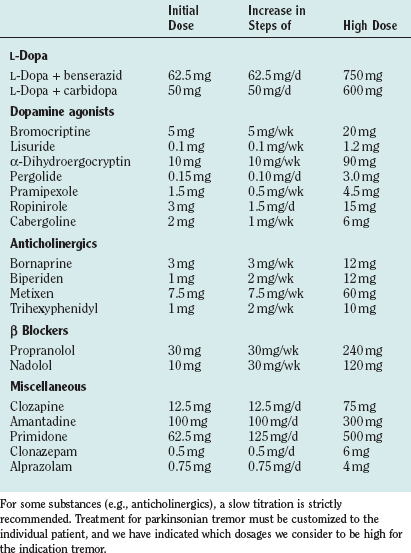
Drug treatments differ for the different forms of tremor in Parkinson’s disease (for drug dosages, see Table 33-4). Our personal approach to the treatment of patients is included in Table 33-5.
L-Dopa is the most effective treatment for the majority of symptoms in Parkinson’s disease. Among the tremors in Parkinson’s disease, mainly the resting tremor is improved, but other forms may also respond. Generally, the effect on tremor is highly variable in patients with Parkinson’s disease, and the tremor may even worsen, especially for the action tremor with frequencies different from the resting tremor frequency. All of the available double-blind studies of different dopamine agonists failed to demonstrate a superior effect of one or the other agonist on tremor, although all of them obviously have a significant effect. For pramipexol, a double-blind-study has shown a favorable effect on tremor.110 Although the treatment of tremors with anticholinergics is often recommended, there are only a few double-blind studies. The anticholinergic bornaprine has been found to be effective in two double-blind studies.111,112 Trihexyphenidyl has been tested alone and compared with amantadine and L-dopa.113 Possible side effects are dry mouth, visual disturbances, constipation, glaucoma, disturbance of micturition, and memory deficits. Especially in elderly subjects, confusional states can occur, which are reversible after cessation of the drug. Discontinuation may induce a severe rebound effect. A study has provided ample evidence that patients treated with anticholinergics have a higher incidence of Alzheimer pathology.114
The favorable effect of clozapine on rest tremor has been confirmed in several studies,115,116 which have shown a good effect on resting tremor—even when other drugs failed.117 No tolerance has been observed over 6 months. The dosage was 18 to 75 mg. Major side effects are sedation and leukopenia as a serious, even lethal complication in some patients.
Functional neurosurgery is a useful treatment for some patients who cannot be treated otherwise. Thalamic thermocoagulation or deep brain stimulation (DBS) of the Vim improves tremor but does not improve akinesia. Lesional surgery cannot be applied bilaterally due to speech disturbances (but DBS can) and therefore is no longer the surgical treatment of first choice.68 Pallidotomy, as well as stimulation of the pallidum, also improves tremor. Subthalamic nucleus (STN) stimulation improves tremor118,119 as well as akinesia and rigidity and is the preferred surgery. Further controlled studies are necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree