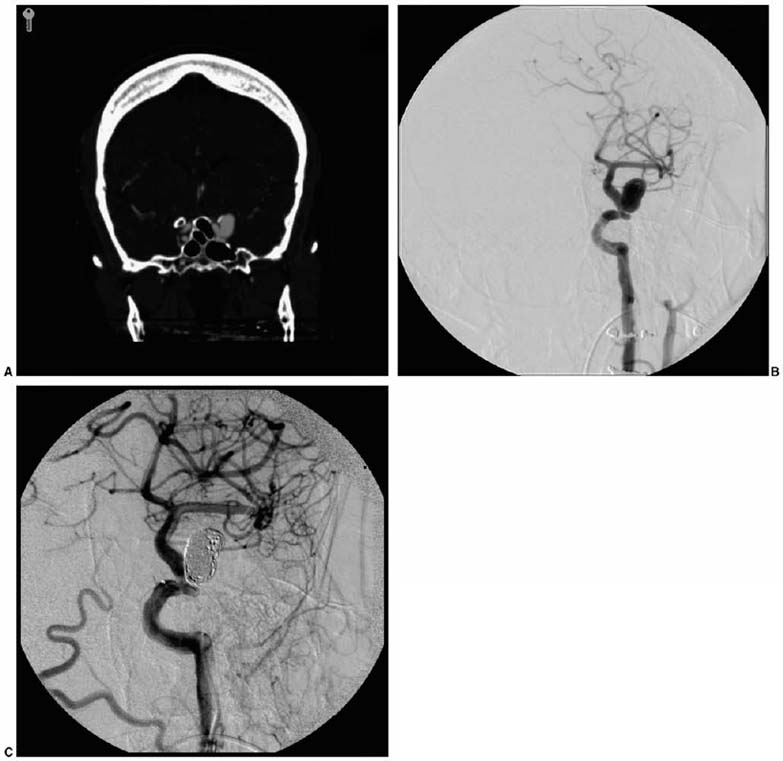
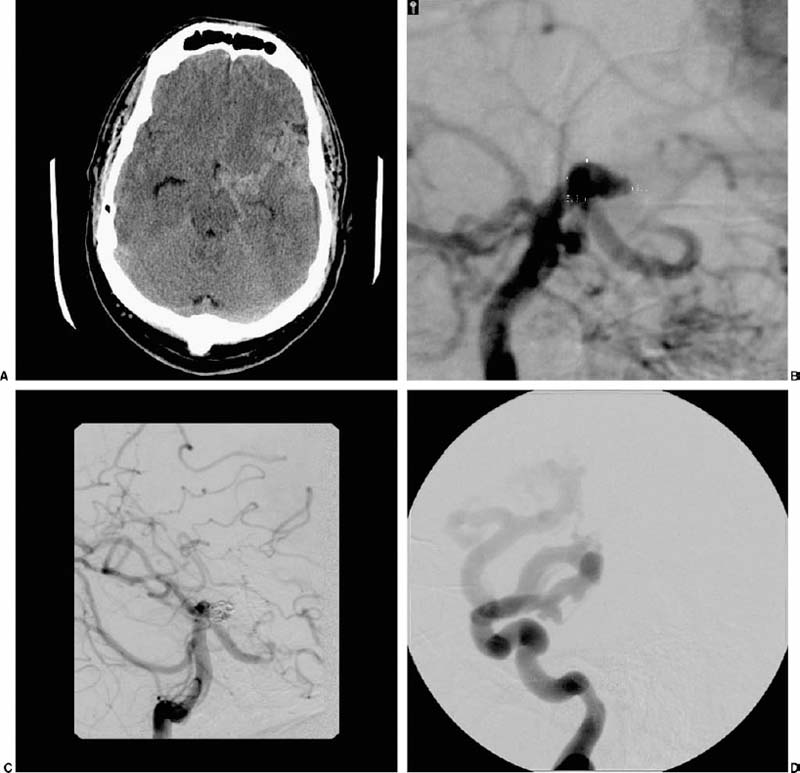
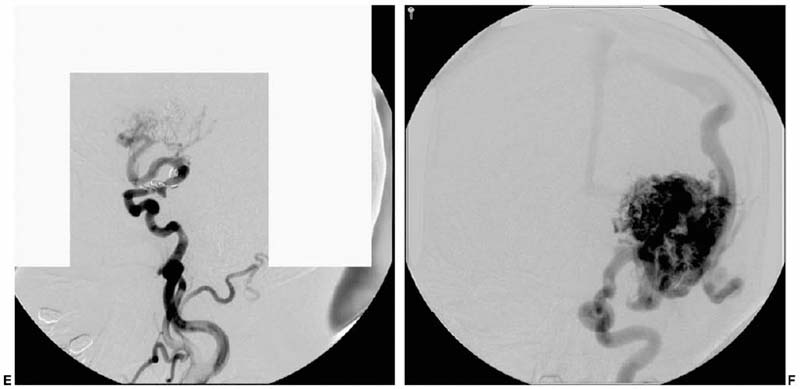
Trends in Neurovascular Surgery Objectives: Upon completion of this chapter, the reader should be able to identify advances in the microsurgical management of aneurysms, arteriovenous malformations, dural arteriovenous fistulas, cavernous malformations, and ischemic diseases of the brain. Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians. Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity. The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp * The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons. The last decade has met with remarkable advances in the surgical treatment of neurovascular disease as well as an improved comprehension of the neurophysiology that underpins its basic science. Many of the advances in clinical neurovascular disease have resulted from the considerable refinements in neuroendovascular techniques, which have paved the way for a variety of new and exciting treatment options for complex diseases of the cerebral vasculature. Terms such as superselective angiography, microcatheter-based embolization, and stent-assisted coiling are now commonplace in both our literature and daily vernacular. While many of us have been quick to educate ourselves about new technologies in an attempt to provide the best possible therapies for our patients, we must adopt these treatments carefully and do so with a degree of cautious optimism. New strategies for the management of disease, while very intriguing and increasingly promising, must measure up to the existing techniques with regard to safety and efficacy to earn a firm place in modern treatment practices. We must strike the proper balance between technical innovation and patient safety. The multitude of recent technological innovations developed for the management of neurovascular disease has created a paradigm shift with regard to our practice environment. A multidisciplinary group of physicians, each possessing a subspecialized training background, is now making the important therapeutic decisions, as opposed to a single individual. The modern neurovascular group is typically composed of neurovascular surgeons, neuroendovascular surgeons, neuroradiologists, neuroanesthesiologists, stroke neurologists, critical care physicians, and physiatrists. By discussing the challenging problems we face in our practices in a collaborative manner, we can consider a variety of different perspectives in a professional setting, thereby facilitating a more integrative approach to disease management. With the increased development of these combined neurovascular groups throughout the world, as well as the costly technology needed to support them, patients with complex neurovascular lesions disease routinely will be referred to large tertiary referral centers for the treatment of their disease. In concert with this recent paradigm shift in practice environment, there is a similar and analogous change in the training of today’s neurovascular surgeon. Several programs, in fact, now provide single fellowships in which proficiency in both “open” surgery and neuroendovascular techniques is acquired. The various pros and cons of such programs are beyond the scope of this chapter, but they are debated actively within the medical community. In this chapter, we attempt to distill the significant recent achievements in each of the principal fields of neurovascular surgery described in this book. This is truly a formidable task and one that might be tackled differently by other authors, as it is subjective in nature. Nevertheless, several important events have occurred in nearly every aspect of neurovascular therapeutics, many of which have affected our current practice. Our goal is to briefly and accurately describe these achievements, illustrate certain points of controversy, and reflect on the historical context in which these recent advances are situated. The treatment of cerebral aneurysms has evolved over the last 10 years more than any other disease in neurovascular surgery. Not only have there been substantial technical advances, including innovative revascularization techniques, improved aneurysm clip design, and more sophisticated metabolic and hypothermic cerebral protection techniques, but also important changes with respect to treatment strategy. Several significant developments in neuroendovascular technology have fueled the refinement in therapeutics for cerebral aneurysms, and the quality of this treatment modality appears to be steadily improving. In fact, the success of endovascular therapy for cerebral aneurysms has resulted in considerable controversy as to the best available therapy for this disease. Microsurgical clipping as the primary treatment of choice recently has been challenged. Still, as we proceed into the 21st century, there remains an important role for open surgery in the treatment of intracranial aneurysms. Interestingly, because of advances in endovascular therapy, the clinical problems treated by open microsurgery at major referral centers for neurovascular disease have become even more challenging and complex in many respects. Sophisticated cranial base surgery techniques and cerebral revascularization procedures are now widely utilized for the treatment of complex aneurysms. This knowledge and skill set, still rigorously studied microanatomically in the laboratory, will continue to play an important role in neurovascular surgery in the future. Currently, a multicenter prospective randomized trial is underway to compare clipping and coiling for ruptured intracranial aneurysms. Although the preliminary 1 year clinical outcome data for endovascular coiling of ruptured aneurysms from the International Subarachnoid Aneurysm Trial (ISAT)1 appears promising, these results must be cautiously interpreted and properly situated in the context of a previously proven durable therapy that has “stood the test of time” with remarkably rare recurrences. Specifically, the ISAT investigators found that, after 1 year of follow-up, there was an absolute risk reduction for attaining a poor outcome of 6.9% in the endovascularly treated patients. However, the rate of rehemorrhage in the endovascular arm was 2.6 times that of the patients who underwent microsurgical clipping. In addition, endovascularly allocated patients were 3.7 times more likely to require an additional treatment procedure for their aneurysm compared with those treated initially with microsurgery. Based on these current data, there are a number of key questions and concerns. Is the early gain in periprocedural morbidity and mortality made by undergoing an endovascular procedure acceptable given the future risk of rebleeding and the profound implications of such an event? What will be the relative morbidity and mortality rates 10 years from now? What are the medical costs incurred by each of the study groups given the need for repetitive follow-up procedures (angiography with or without additional coiling) in endovascularly treated patients? Poor surgical results, published in the interim ISAT report, have not been borne out in other large, multicenter trials.2 Only approximately 22% of all patients admitted to participating hospitals were ever randomized to the ISAT, a factor clearly leading to inherent selection bias, and one that will ultimately impair the extrapolation of this outcome data to daily practice. Despite the known low incidence of complete aneurysm occlusion and the high risk of recurrence at 1 year with endovascular treatment, no follow-up angiography was required or reported at 1 year. How many of the “good outcome” endovascular patients harbor incompletely occluded or growing aneurysms that will manifest later? In addition, the ISAT trial is Europe based; what is the data applicability to North American centers with high levels of subspecialty staffing? In a differently designed, yet valuable study, Murayama and colleagues3 reported the results of the endovascular treatment of 818 patients with 916 aneurysms at the University of California-Los Angeles using Guglielmi detachable coils (GDCs). Their study was retrospective and nonrandomized; however, it is a candid and detailed account of the safety and efficacy of aneurysm coiling (in both ruptured and unruptured lesions) in a large number of patients by leaders in the field, with multiyear follow-up. Interestingly, these authors found that complete occlusion was achieved in only 55% of the aneurysms treated. In addition, the overall recanalization rate approached 21%. Even more compelling, however, was the discovery that for aneurysms considered ideal for microsurgical clipping (i.e., small aneurysms with small necks), the rate of complete obliteration with coiling was only 75%. These figures stand in stark contrast to a microsurgical series in which a remaining aneurysmal neck after surgery is estimated to be 3 to 6%.4,5 These data have not only been revealing but have forced neurovascular specialists to consider the natural history and optimal management of a posttreatment aneurysmal remnant. For instance, what is the risk of bleeding from an incompletely obliterated aneurysm? Is the risk of future hemorrhage from an aneurysm remnant dependent on the type of previous treatment or the location and morphology of the aneurysm? Finally, how frequently should asymptomatic angiographic recurrences be followed, and by what imaging modality? Enabled by the endovascular advances in the past decade, we now commonly see aneurysms treated with a combination of modalities in a so-called hybrid approach. It is not uncommon for a complex, ruptured aneurysm of the posterior circulation to be incompletely coiled purposefully to prevent early rebleeding, with the plan of treating the lesion with microsurgical clipping once the major risk period for developing edema or vasospasm has elapsed. Conversely, some lesions are clipped intentionally so that a small remnant remains, particularly in cases where complete access to the neck is obscured by vital perforating arteries or skull base structures. In such cases, the remnant is coiled postoperatively to complete the aneurysm occlusion. We have successfully implemented this strategy in the management of complex paraclinoidal aneurysms in which a portion of the lesion extends into the cavernous sinus, but the fundus is in the subarachnoid space. In concert with the greater application of endovascular therapy, neurovascular surgeons will need to manage patients with previously coiled aneurysms. These lesions pose a unique set of challenges that have only recently been appreciated and described in the literature.6,7 The treatment of these patients, while not uniform and clearly multifactorial, can be broadly dichotomized based on the time and nature of recurrence. In patients who acutely recanalize following coiling, temporary trapping followed by aneurysmorrhaphy and coil evacuation often enables straightforward clip reconstruction and exclusion of the aneurysm. Such a strategy, however, is significantly more difficult to carry out in patients who recur more than 6 months after coiling. By this time, the coils have become firmly adhered to the fundus of the aneurysm and may extrude from the aneurysm tissue itself. The coil mass frequently stents open the residual aneurysm wall, making gathering of the sac and parent artery reconstruction quite challenging even with prolonged periods of temporary occlusion. In these “chronic” coiling patients presenting with asymptomatic recurrence, we therefore prefer to wait for the remnant to expand enough so that a surgically clippable neck is present and the parent artery is more amenable to reconstruction. The risk of hemorrhage during this time is unknown. In relation to aneurysm therapeutics, the management of lesions that cannot be clipped has substantially evolved recently (Fig. 1-1A-C). Parent cerebral artery sacrifice for the treatment of complex cerebral aneurysms was used and refined by Dr. Charles Drake8–10 in the early 1990s; it is still used successfully today. This treatment is now being compared with attractive endoluminal strategies of parent artery reconstruction made possible by the development of flexible intracranial stents.11,12 Aneurysms with no reconstructible neck are treated from the inside out, with an endoluminal stent serving to reconstruct the parent vessel as well as to prevent herniation of the coil mass out of the aneurysm sac. Despite the numerous theoretical advantages of this approach and a multitude of successes, reports of aneurysm recurrence, thromboembolic ischemia, parent vessel thrombosis, and subarachnoid hemorrhage following the application of stent-based therapies currently exist.13–17 Moreover, some concerns exist with regard to the need for potent antithrombotic regimens in patients treated with a stent who have a ruptured aneurysm. In cases of incomplete aneurysm occlusion with a stent, does the patient on powerful antithrombotic agents have an increased risk of rebleeding? Is the outcome significantly worse if this patient does bleed again? These controversies notwithstanding, we are very much encouraged by this novel way to treat aneurysms untreatable by conventional clipping methods; we look forward to future developments in this methodology as well as additional studies assessing its utility. Several additional areas of development, both surgical and conceptual, exist today in the management of cerebral aneurysms and deserve mention. In concert with the profound advances in neuroimaging, surgical treatment of cerebral aneurysms using computerized tomographic angiography (CTA) alone in preoperative planning has been recently described. Despite its controversy, experienced neurovascular teams have documented the feasibility of this approach. In cases of the poor-grade or moribund aneurysmal subarachnoid hemorrhage patient possessing a concomitant life-threatening intracerebral hematoma, surgery based on only CTA prior to catheter angiography may prove life saving by avoiding the delay imposed by angiography. Indeed, we treat the majority of our patients with unruptured aneurysms and an increasing number of patients with ruptured lesions without cerebral angiography. It is our opinion that cerebral angiography will become the province of the endovascular surgeon and angiography will be performed only when endovascular intervention is considered or for follow-up after intervention. Current trends include a recognition of the value of screening asymptomatic patients who demonstrate identifiable risk factors for harboring a cerebral aneurysm and who have a higher risk of rupture, such as family history, smoking, hyperlipidemia, coronary artery disease, or inherited conditions associated with severe vasculopathies such as autosomal dominant polycystic kidney disease or Marfan syndrome. We still must identify the overall benefits of screening patients at risk, the modality of choice, and the time of initial evaluation as well as follow-up. Also, if an aneurysm is detected in a patient in a high-risk category, should data from the International Study of Unruptured Intracranial Aneurysms (ISUIA)2 be applied to treatment decisions, or is this data not applicable in the high-risk patient population? Several important questions in terms of aneurysm natural history and screening deserve critical analysis in the decade to come. FIGURE 1-1 This is a 52-year-old right-handed female who presented with sudden-onset headache following exercise. (A) Coronal CT angiographic reconstruction reveals an aneurysm arising from the petrous carotid artery, which extends into the subarachnoid space. Because of the relative inaccessibility of the neck surgically an endovascular strategy was pursued. (B) Digital subtraction angiography shows the petrous aneurysm. (C) Postcoiling angiography demonstrates complete occlusion of the aneurysm with preservation of the afferent and efferent arterial circulation. Cerebral arteriovenous malformations (AVMs), lesions composed of a dynamic array of arteries and veins linked by a central nidus, are among the most challenging entities in all of neurovascular surgery. A key review of the unique practice of Professor Troupp19 helped to clarify the behavior of symptomatic AVMs of the brain in the absence of treatment, revealing a 4% annual risk of hemorrhage. Each hemorrhagic episode was shown to be associated with an approximately 10% risk of death and a 30% risk of major neurological morbidity.20 With the potentially severe natural history associated with an untreated cerebral AVM in mind, several significant advances have been made in the neuroendovascular and neuroimaging arena as well as in radiosurgical methods. These have resulted in a greater breadth of available treatment options for simple as well as the most complex cerebral AVMs. The modern treatment of AVMs, particularly large lesions embedded within eloquent tissue, has truly evolved into a multidisciplinary effort in which several treatment strategies are integrated synergistically to render the best possible intervention. A broader range of AVMs is now curable with better safety than was possible a decade ago. The most valuable current application of endovascular therapy in the treatment of AVMs is goal-directed embolization of arterial feeders prior to either operative resection or, much less commonly, radiosurgical obliteration (Fig. 1-2A-F). Initially pioneered by Luessenhop in the 1960s,21,22 the application of selective endoluminal devascularization of arterial inflow to a large AVM can frequently allow a safer and less time-consuming micro-surgical resection. Preoperative embolization of deep arterial feeders that are accessible only with significant parenchymal retraction now is possible using softer, smaller, and more trackable microcatheters, safer microwires, and better embolic agents. Super-selective intranidal embolization techniques when compared with earlier-generation flow-directed prenidal arterial occlusion, are a dramatic improvement in the treatment of complex AVMs. Many complex AVMs that would be otherwise too formidable for safe microsurgical resection alone are now treatable through this combined approach. Moreover, preembolization significantly reduces the incidence of postoperative hyperperfusion and intracranial hemorrhage. In fact, the potential benefits of preoperative embolization may have created an environment in which the technique is over-applied. In general, the use of embolization as a preoperative adjunct should be restricted to situations in which the risk of microsurgery and embolization combined is less than the risk of microsurgery alone. In stating this, preembolization is usually considered unnecessary for the majority of grade I and II AVMs, which can be treated with microsurgery alone, yielding excellent results.23,24 In addition to its role as an adjunct, embolization is used as a curative measure for certain small AVMs that are fed by a limited number of accessible arterial pedicles. In choosing embolization as either a preoperative adjunct or a stand-alone therapy, the neurovascular surgeon must be keenly aware of the wide spectrum of possible complications, which include but are not limited to stroke, hemorrhage, arterial dissection or perforation, and death. Taylor and colleagues25 recently reported the complications they accrued over an 11-year period in the embolization of 201 patients with cerebral AVMs at the University of Texas Southwestern Medical Center in Dallas. These data revealed a 2% risk of death and 9% risk of permanent neurological morbidity, comparable figures to those of other large, well-respected centers. A current point of controversy is the role of embolization as a palliative measure for symptomatic AVMs that are not safely treated with complete excision and are too sizeable for radiosurgical obliteration. The reduction of flow may, in some cases, reduce the mass effect and arterial steal associated, with large, high-flow AVMs. Despite this possibility, we would argue against the common practice of palliative embolization as there is no literature available to suggest a better outcome. In fact, several reports suggest that staged embolization without complete AVM obliteration may alter the nidal hemodynamics in such a manner that an increased risk of hemorrhage is produced.26,27 In the face of the significant risks associated with embolization therapy, a persistent and possibly increased risk of future hemorrhage is simply not acceptable in the overwhelming majority of cases. In addition to the increasing role of endovascular techniques in the treatment of cerebral AVMs, stereotactic radiation has also proven very useful in properly selected patients. Radiosurgery, which may be delivered by gamma knife, a linear accelerator, or heavy charged particle, has been used successfully both alone and in concert with other therapeutic options for many years. With its initial introduction as a treatment modality for cerebral AVMs by Steiner in 1972,28 the avoidance of potential morbidity and mortality associated with a craniotomy was appealing to both patients and physicians alike. When the AVM is deep within subcortical gray matter, radiosurgery may be the only rational therapeutic choice available. Several decades later, we now have a greater (but still incomplete) understanding of the various advantages as well as disadvantages of this treatment strategy, as well as its role in the treatment of AVMs. In radiosurgical therapy for an AVM, there is a 2- to 3-year latency period during which time the patient is still at risk of hemorrhage. In addition, there have been numerous reports of angiographic recurrence of cerebral AVMs following previously documented radiosurgical obliteration29,30; this is an extremely uncommon event after angiographically verified microsurgical excision. Further, the development of postradiosurgery neoplasms (particularly meningiomas) has also been demonstrated.31,32 This certainly impacts treatment decisions in children and young adults with AVMs who have an otherwise normal life expectancy. Radiosurgery, while clearly efficacious for certain AVMs, requires further study with regard to the long-term effects of radiation to neighboring parenchyma, the incidence and clinical effects of potentially subtle neuropsychological sequelae of radiation, and the true risk of later recurrence with clinically significant hemorrhage or neurological decline. The size of an AVM as well as the radiosurgical dosage utilized, are integral factors in predicting the overall success of radiosurgery. An angiographic cure can be expected in up to 85% of patients harboring lesions with a diameter smaller than 3 cm. Larger lesions, which have a poor rate of obliteration with a single radiosurgical treatment, have been approached with either preradiation embolization or staged radiosurgery. Both of these approaches lack long-term efficacy data. While we have used both approaches in our practice, we have applied them very selectively. Endovascular embolization followed by radiosurgery is limited by the potential for subsequent arterial recanalization in regions of an AVM previously considered to be obliterated.

 Aneurysms
Aneurysms
 Arteriovenous Malformations
Arteriovenous Malformations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


