Myelopathy
Pain
Miscellaneous
Sensory disturbances
Band-like chest pain
Multiple sclerosis symptoms
↑ or ↓ skin sensitivity
Axial thoracic back pain
Spinal neoplasm/tumor symptoms
Hypoesthesia
Axial lumbar pain
Psychiatric disorder symptoms
Paresthesia
Periodic lumbago
Demyelinating disease symptoms
Anesthesia
Spinal pain
Ovarian disorder symptoms
Complete sensory deficit
“Labor-like” pain
Reproductive disorder symptoms
Genital anesthesia
Radiculopathy
Cardiac disorder symptoms
Dysesthesia
Pain accentuated by cough
Symptom intermittency
Motor disturbances
Point tenderness
Severe headaches
Abdominal reflex loss
Pure axial back pain (absent other symptoms)
Spontaneous intracranial hypotension
Hyperreflexivity
Pure radicular pain (absent other symptoms)
Accompanying scoliosis
Abnormal reflexes
Abdominal pain
Accompanying kyphosis
Diffuse weakness
Intercostal neuralgia
Resultant from trauma
Mono/paraparesis
Radiculomyelopathy
Paraspinal muscle rigidity
Mono/paraplegia
Angina pectoris
Sphincteric changes
Brown-Séguard syndrome
Groin pain
Bladder dysfunction
Rapid-onset paraplegia
No pain
Bladder urgency
Progressive paraplegia
Bowel dysfunction
Spastic, ataxic gait
Potency disturbances
Lumbar neurologic symptoms
Trophic disturbances
Gallbladder disease
Gastritis
Renal calculi
Patients with symptomatic TDHs indicated for surgical intervention present with some combination of pain (back, thorax, radicular) paired with myelopathy and/or bladder or bowel dysfunction that are consistent with MRI findings [5–8]. Following confirmatory diagnosis of TDH, surgical treatment requires consideration of disease morphology (central vs. lateral, calcific vs. soft) which, historically, has defined the specific approach (orientation and exposure) and procedure (instrumented or not) used [9, 10], though surgeon comfort and preference with the proposed surgical approach are also important factors [11].
Early surgical treatment of symptomatic TDH included decompressive laminectomy, which was largely abandoned following high reported rates of procedural morbidity resultant from transdural exposures and spinal cord mobilization [4–6, 11]. This highlighted the need for direct-access approaches to the pathology with minimal spinal cord manipulation. From decompressive laminectomy, costotransversectomy, transthoracic (thoracotomy), and thoracoscopic approaches were subsequently developed for the treatment of TDH. Costotransversectomy, while more effective than decompressive laminectomy, is challenged by limited access to contralateral disease and still requires spinal cord retraction (especially in cases of central, calcific discs or those requiring osteotomies), with rhizotomy required in most cases [12]. Thoracotomy provides the “gold standard” exposure to treat a variety of herniations, including giant, central, and calcific discs [4, 13, 14]. However, thoracotomy is becoming more scrutinized in modern practice (though still regularly used) due to the substantial procedural morbidity, including pulmonary complications related to single-lung, dual-lumen intubation and the persistence of post-thoracotomy pain and pain syndromes secondary to the incision. In one study, postthoracotomy pain was present in 50 % of patients after surgery, with 30 % of patients reporting continued painful symptoms 4–5 years postoperatively [15]. Thus, thoracoscopic approaches began to be used to minimize the morbidity of thoracotomy. While thoracoscopy is effective in minimizing the exposure-related morbidity of thoracotomy, procedural implementation is challenging with a long learning curve (visualizing a three-dimensional working space in two dimensions), need for highly trained staff, and equipment expense that has limited its utility [16–18]. Also, procedural morbidity remains a consideration in thoracoscopic approaches, with pulmonary complications still a concern with the use of single-lung intubation and the need for emergent conversion to thoracotomy in the case of major intraoperative complications unable to be managed through the thoracoscopic exposure (vascular injury).
TLJ and Thoracic Neoplasm
While cancer in the spine as a primary tumor site is relatively rare, metastatic disease to the spine is remarkably common, with more than half metastasizing from the breast, lungs, or in lymphoma [19]. With the spine being the most common osseous site of tumor metastases, as many as 30–90 % of patients who die due to cancer have spinal tumors present [19–21]. Those with symptomatic spinal disease resulting from tumor presence, however, are less common. Spinal cord compression in spinal neoplasms has been reported in as little as 5 % to as many as 40 % of cases, with only 10–20 % of those exhibiting symptoms requiring surgery [20]. This results in approximately 25,000 cases of surgery for spinal tumors a year in the USA [20], with the majority in the thoracic spine. With 1.4 million new cases of cancer diagnosed in the USA each year, and half of those eventually succumbing to the disease or related complications, and these numbers projected to increase, effective and expedient treatments are needed to manage the growing problem [21, 22].
The treatment of spinal neoplasms is complicated by many factors. Factors impacting surgical decision making include the tumor type and biology (malignant, benign, aggressive, etc…), presence of multiple or peripheral metastases, control of systemic metastases, general health and physical condition of the patient, location in the spine (cervical, thoracic, lumbar, sacral), portion of the vertebra(e) involved (anterior and/or posterior element or dural involvement), symptomology (pain most common), the presence and severity of neural compression, pending negative neural or structural changes, the extent and type of prior or concurrent adjuvant therapies (radiation therapy, radiosurgery, chemotherapy, etc…), and life expectancy [19, 21, 23, 24]. This leads to the requirement for both a multi-disciplinary and individualized care plan developed between neurosurgery and/or orthopaedic surgery, medical and radiation oncology, radiology, and rehabilitation services. This is all in combination with a growing need for integration of less-invasive approaches with decreased morbidity in these patients susceptible to complications to allow for a hastened recovery and return to adjuvant therapies [21, 25] and quality of life.
Similar to treatment of TDH, surgical approaches include the gold-standard anterior approach (majority of tumors lie within the anterior column) through thoracotomy, thoracoscopic approaches (for smaller, contained anterior tumors), and posterior approaches (costotransversectomy) alone for posterior-based tumors or in conjunction with an anterior approach for tumors affecting the entire segment. The challenges of each approach are similar to those previously stated, though with the increased potential for infection and wound healing issues when using open-exposure approaches in these immunocompromised patients, especially considering the more extensive nature of pathologic excision compared to that involved with THD (typically corpectomy).
Thoracolumbar Trauma
Traumatic spinal fractures most commonly occur around the TLJ, as the thoracic cavity superior to this watershed area creates a pivot point that results in an elevated mechanic risk. In fractures requiring surgical intervention (e.g., unstable fractures and/or cases with neural/pending neural deterioration), corpectomy and fixation is the standard treatment with, similar to the previous indications, anterior, lateral, and posterior options, each with its own set of benefits and drawbacks (Table 24.2).
Table 24.2
Surgical approaches for thoracolumbar corpectomies and the treatment of thoracic disc disease
Anterior (anterolateral) approaches | Posterior approaches | Posterolateral approaches |
|---|---|---|
Transthoracic extrapleural | Laminectomy | Transpedicular |
Transthoracic transpleural | Transpedicular facet sparing | |
VATS | Costotransversectomy | |
Mini-open lateral extrapleural | Transforaminal | |
Mini-open lateral transpleural | Transfacet pedicle sparing | |
Lateral extracavitary |
Mini-Open Lateral Approach Background and TLJ/Thoracic Utility
The minimally disruptive lateral transpsoas approach for anterior lumbar interbody fusion (extreme lateral interbody fusion, XLIF®, NuVasive, Inc., San Diego, CA) was developed in the late 1990s and early 2000s by Luiz Pimenta, M.D., Ph.D., and introduced into the literature in 2006 [26]. The procedure was developed as a less-invasive alternative to direct anterior approaches with many of the associated complications avoided. As the lumbar procedure became more widely adopted, the approach and procedure’s use were expanded into the TLJ and thoracic spaces, with the benefits from the lower translated into the upper spine for, generally, more complex indications including TDH, tumors, and traumatic fractures. The procedure allows for access to the lateral aspect of the thoracic and thoracolumbar spine through a small (approximately 3–6 cm, depending on whether or not a corpectomy is being performed) incision with surgical access similar to that of a thoracotomy, though through a smaller exposure window. The exposure also, generally, allows for exposure of the ipsilateral posterior elements, expanding the variety of feasible treatment pathology, particularly with spinal tumors. The approach also provides for maintained inflation of the ipsilateral lung, avoiding the complications of dual-lumen intubation.
The remainder of this work will focus on technical considerations and reported outcomes of the mini-open lateral approach in these more advanced thoracolumbar applications.
Review of Anatomy/Special Technical Considerations
Thoracolumbar Junction
At the TLJ, the most relevant anatomy to the lateral approach are the diaphragm and the inferior border and aspect of the pleural sac (depending on level treated) [27]. The diaphragm is a musculotendinous sheet that divides the abdominal and thoracic cavities. The diaphragm attaches in three places, classified as anterior (sternal), lateral (costal), and posterior (lumbar) attachments. Management of the diaphragm includes consideration for preservation of the diaphragm at upper lumbar levels (L1, L2), deflecting it superiorly during exposure of the retroperitoneal cavity (Figs. 24.1a–e). At the TLJ and lower thoracic levels, where the diaphragm may not be able to be mobilized without violation for adequate lateral disc space exposure, a transdiaphragmatic approach can be used, often with blunt dissection through the layer and no closure needed upon removal of the retractor. Other anatomy to consider are the lower ribs, although upper lumbar levels are generally approachable without any rib resectioning, sometimes assisted through the use of angled procedural instruments, or exposure can be obtained between the ribs using the split-blade retractor (Ma Xcess®, NuVasive, Inc.).
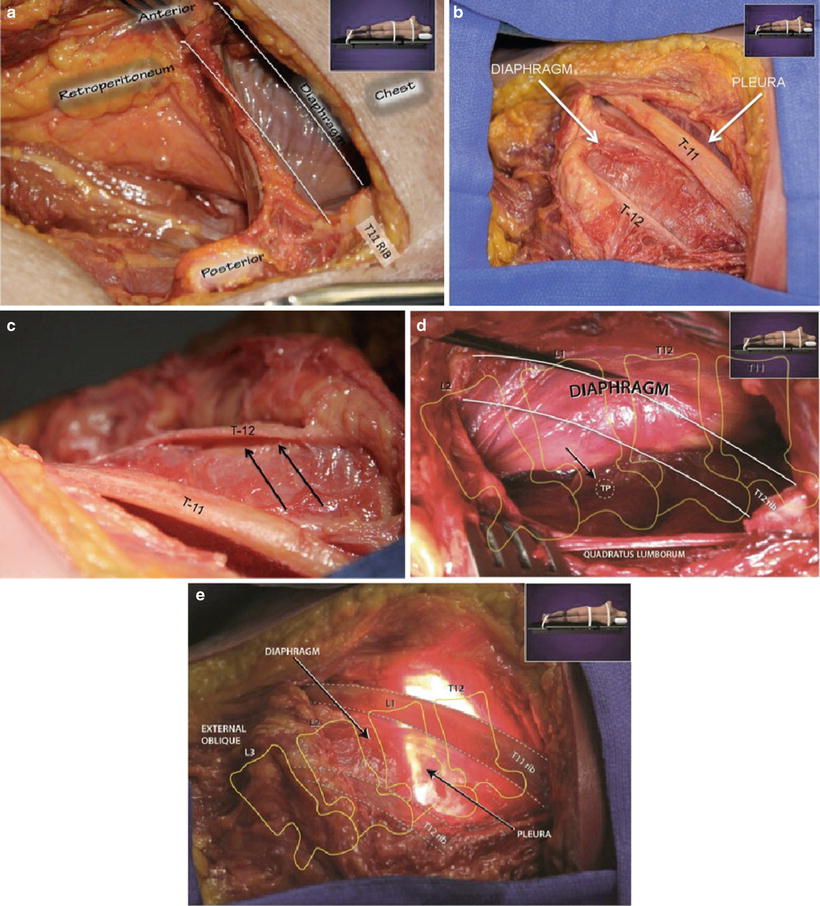

Fig. 24.1
(a) Cadaveric specimen in the right lateral decubitus position with the chest/abdominal wall removed demonstrating how the diaphragm separates the thoracic cavity from the abdomen. The 11th rib has been resected and is outlined. (b) Cadaveric specimen in the right lateral decubitus position demonstrating the relative relationship of the diaphragm and pleural cavity (arrows) to the 11th and 12th ribs. (c) Cadaveric specimen demonstrating the costal attachments (arrows) of the diaphragm to the medial surface of the 12th rib when looking from cranially to caudally. (d) Cadaveric specimen in the right lateral decubitus position demonstrating the posterior or lumbar attachments of the diaphragm to the transverse process of L1. The resected rib has been outlined in white lines and the arrow is indicating the intervening point between the medial and lateral arcuate ligaments. TP transverse process. (e) Cadaveric specimen in the right lateral decubitus position demonstrating the relative relationship of the diaphragm and illuminated pleural cavity (arrows) to the 11th and 12th ribs
Thoracic
In the thoracic spine, the primary factors relevant to the approach are the ribs and neurovascular bundle, pleural cavity, and rib head at the costal facet. The neurovascular bundle runs along anterior-inferior aspect of each rib and should be considered during both the initial approach and during suture for preservation. Once the superficial exposure has been made, the pleural cavity can either be managed through a transpleural or retropleural approach [28]. The retropleural approach involves careful mobilization of the pleura from the chest wall and generally obviates the need for a chest tube postoperatively. Finally, the rib head will be a landmark for thoracic levels and must be considered at each level being treated for identification of the posterior disc border, pedicle, and dural sheath. Also, it will need to be resected in most cases, especially at upper thoracic levels, to gain adequate exposure the lateral disc space.
Despite some differences in regional anatomy, interbody and corpectomy technique and instrumentation at all levels (once exposed) generally follow conventional practice and principles, though through a lateral working window.
Surgical Technique
As previously mentioned, the surgical technique for minimally disruptive lateral interbody fusion was first published in the literature in 2006, followed by several detailed technical descriptions of the lumbar and more advanced applications, the most recent being from 2013 [29]. The mini-open lateral technique for TLJ/thoracic corpectomy (trauma and tumor) and thoracic disc degeneration have also been previously described [11, 27, 28, 30–38], and largely serve as the foundation for the current technical description.
Indications and Limitations
Surgical indications for the mini-open lateral approach at the TLJ and in the thoracic spine include treatment at any level requiring a corpectomy, discectomy, and/or fusion from approximately the T5 vertebra (limited by the scapula) inferiorly to the TLJ. For traumatic pathologies or tumors extending to the posterior elements, a combined anterior (lateral) corpectomy with vertebral body replacement (VBR) and posterior approach for decompression and fixation can be used. If the lesion is isolated to the anterior column only or anteriorly as well as within the ipsilateral posterolateral bony anatomy and without instability requiring posterior instrumentation, the lateral approach alone may be used (Fig. 24.2a–e). A relative limitation to the approach includes anatomy that interferes with the lateral approach (e.g., variant position of the great vessels).
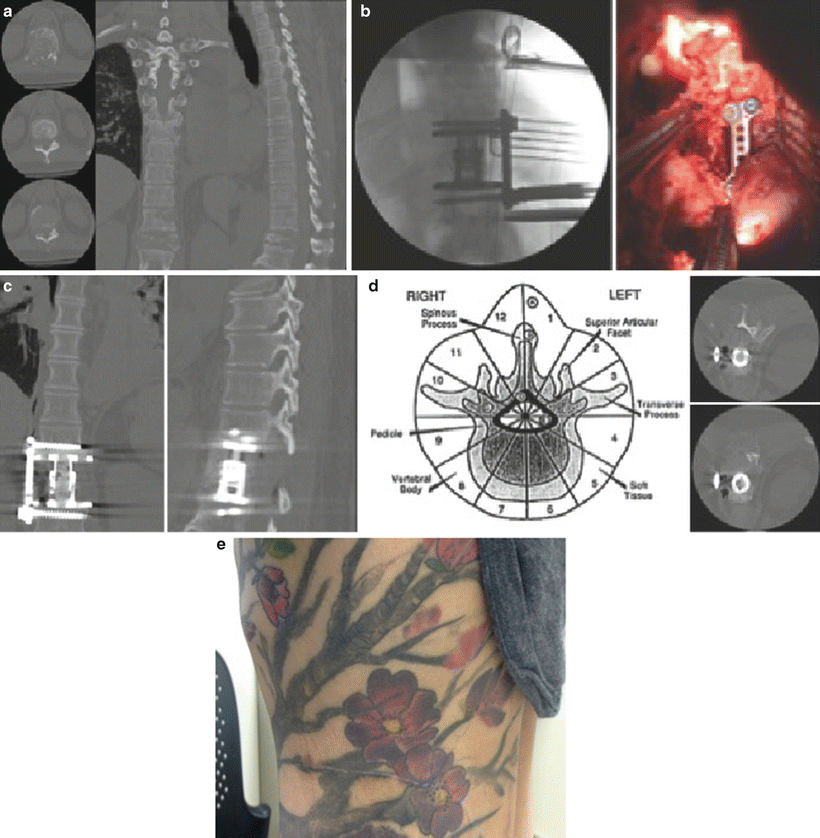

Fig. 24.2
Preoperative computed tomography (CT) showing T11 metastasis in the vertebral body and pedicle (a) treated with a retropleural approach for lateral corpectomy, laminectomy, and facetectomy (b) following by placement of wide footprint expandable cage with anterolateral plating (b, c). Postoperative axial CT shows area of decompression (approximately zones 4 through 11 on the Weinstein Boriani Bagini (WBB scale)) available through the mini-open lateral approach for corpectomy (d). (e) Shows postoperative cosmesis following mini-open lateral corpectomy
Preoperative Considerations
While this and other minimally disruptive surgical approaches have both special intraoperative considerations and allow for early postoperative mobilization, care must be taken for proper pre-, intra-, and postoperative analgesia and anesthesia [39].
In cases where intraoperative electromyography (EMG) is used (e.g., TLJ and lower thoracic (T8–T11 segments)), anesthesia should limit the use of muscle relaxants and paralytics and, if needed for induction or other reasons, only short-life span varieties should be delivered to allow for unfettered EMG readings during the procedure. Otherwise, standard intraoperative neuromonitoring for these cases will include monitoring somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs). As such, for MEPs, total intravenous anesthesia (TIVA) (no inhaled anesthetics) is required.
Some specialized equipment required for the mini-open lateral approach include a radiolucent surgical table capable of both a bendable break and tilting in the ventral–dorsal and cranial–caudal orientations, fluoroscope (C-arm), access system and peripherals (MaXcess®, NuVasive, Inc.), general anterior and lateral procedural instruments, neuromonitoring platform (NV M5), and bipolar electrocautery.
Surgical Considerations
As has been previously reported [26, 29, 40], there are five key steps for an efficient and reproducible lumbar XLIF. These steps include (1) Careful patient positioning, (2) Gentle retroperitoneal development and dissection, (3) Meticulous psoas passage using advanced neuromonitoring integrated into approach and procedural instrumentation, (4) Adequate discectomy and proper endplate preparation, and (5) Appropriate interbody implant sizing and placement. These tenets largely remain relevant in thoracic and TLJ indications, modified to three key steps: (1) Careful patient positioning, (2) Gentle supra- or infra-diaphragmatic and/or retropleural plane development (if not using a transthoracic approach), and (3) Adequate discectomy/corpectomy with placement of appropriate instrumentation (intervertebral cage(s), VBR devices, and/or supplemental internal fixation).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








