Fig. 10.1
A 7-mm right posterior communicating artery UIA with a favorable neck to dome ratio (a) treated with unassisted coil embolization (b)
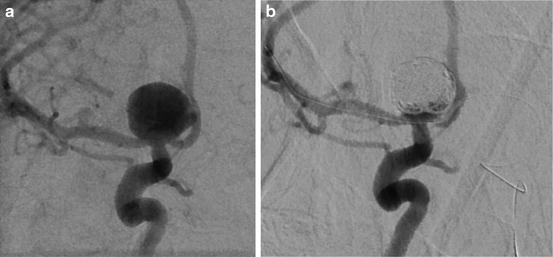
Fig. 10.2
A 1-cm right carotid terminus UIA with a wide neck (a) treated with balloon-assisted coiling (b)
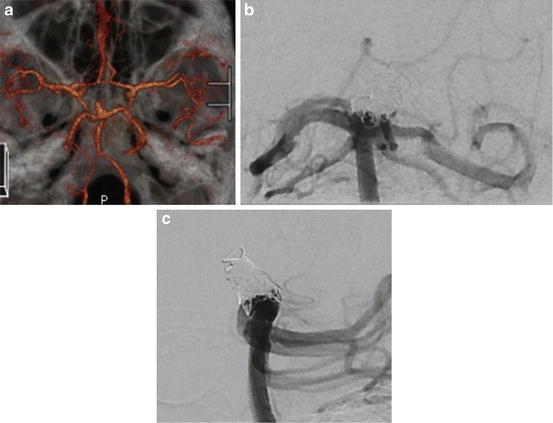
Fig. 10.3
A wide-neck basilar tip UIA (a) treated with P1 to P1 ENTERPRISE stent-assisted coil embolization. Shows coils conforming to outer surface of the stent on anterior–posterior (b) and lateral (c) projections
Giant Aneurysms
In one of the earlier reports on coil embolization of GIAs, 69 % of aneurysms were incompletely occluded at 6-month follow-up angiography (range, 1–11 months). These recurrences have been attributed to thrombus dissolution, coil compaction, or coil migration into preexisting thrombus. GIAs frequently require retreatment, sometimes more than once [43]. Even the use of BAC and SAC has not adequately addressed these high rates of recanalization [44, 45].
Liquid embolics
Liquid embolic agents, such as Onyx HD-500, were introduced in an attempt to reduce the high recanalization rates seen described above. The CAMEO trial (Cerebral Aneurysm Multicenter European Onyx) was the largest study on the use of Onyx HD-500, the only FDA-approved liquid embolic agent for the treatment of intracranial aneurysms, as the primary curative strategy. Out of the 100 aneurysms treated, 19 were classified as GIAs. All had complete (100 %) or subtotal (90–99 %) initial occlusion. Of the 19 GIAs, one (5 %) was reported to need retreatment for recurrence at 3-month follow-up angiography. However, four patients did not have 3- to 6-month follow-up outcomes reported, and three patients had complete or subtotal occlusion that progressed to incomplete or indeterminate occlusion [46].
Liquid embolic delivery requires balloon inflation across the aneurysm neck. The balloon must remain inflated for at least 3 min after injection of the embolic agent, allowing the agent to precipitate inside the aneurysm. If the balloon is let down too soon, the liquid embolic agent can leak into the parent artery causing embolic occlusions. Additionally, the embolic agent can leak into the parent artery around the balloon if it is not inflated adequately, yet if inflated too much, arterial dissection or rupture can occur with devastating consequences. In the CAMEO trial, procedure- or device-related permanent neurological deficits, including hemorrhage (ICH), ischemic stroke, or worsening cranial nerve deficit, were present in 8/97 patients, and seven died, two of which were procedure related (one groin complication and one ICH from vessel dissection), one death was disease related, and four others were from unrelated causes.
These risks, along with concerns about long-term durability, have limited the use of liquid embolic agent use to treat GIAs.
Parent vessel occlusion
Parent vessel occlusion (PVO) to treat GIAs of the carotid artery dates back to the eighteenth century when it was first described by Cooper in 1809 [47]. From historical experience on surgical clamp occlusion, approximately 75 % of patients can tolerate surgical clamp occlusion of the ICA [48]. Surgical PVO has been discussed earlier in this chapter, and we will further discuss endovascular treatment here.
PVO can be performed by several techniques including coil embolization and/or Onyx HD-500 embolization. In most patients with unruptured aneurysms presenting with mass effect on the cranial nerves, symptoms are improved soon after therapy [48]. Complications from endovascular PVO therapy for GIAs include: (a) increased local mass effect from aneurysm thrombosis or the devices used to achieve aneurysm occlusion, (b) subarachnoid hemorrhage, and (c) stroke from thromboembolic events. In a series of 15 patients undergoing endovascular PVO one patient developed new sixth cranial nerve palsy, three patients had access site complications, and one died from aneurysm rupture [49].
Due to the recent developments in endovascular technology, there is a paradigm shift toward vessel-preserving treatments such as the use of flow-diverting stents.
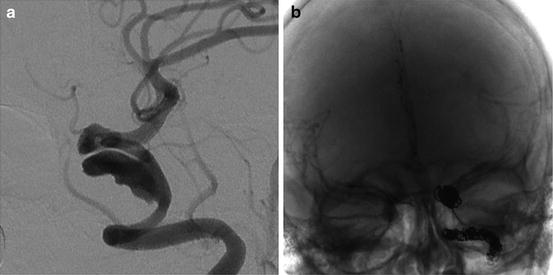
Illustrative case 4: A 45-year-old woman presented with severe headache and ophthalmoparesis progressing rapidly to ophthalmoplegia. She underwent diagnostic angiography that showed a large partially thrombosed cavernous carotid artery aneurysm encompassing a large portion of the parent vessel (Fig. 10.4a). Given the difficulty in treating the aneurysm with vessel-preserving strategies and progressive nature of her symptoms, it was decided that parent vessel sacrifice should be considered. She underwent balloon test occlusion with hypotensive challenge without neurological deficits (see Chap. 16). Robust filling through the anterior communicating artery was observed. Venous phase delay was <1 s. She subsequently underwent successful vessel sacrifice with long-term near-total resolution of her ocular symptoms (Fig. 10.4b).
Flow diversion
Flow diversion is a highly promising, emerging technology that was initially developed to treat aneurysms with morphologies not amenable to coil embolization. Flow diversion results in disruption of flow near the aneurysm neck, inducing thrombosis in the aneurysm sac while keeping the physiological blood flow in the parent vessel and adjacent branches intact.
Reports on the use of the PED include aneurysms of all sizes. A literature review on the outcomes of aneurysmal flow diverters showed immediate angiographic aneurysm occlusion in only 8–21 % of patients [36-7, 50]. However, over time the aneurysm occlusion rates were higher than conventional treatments with complete occlusion ranging from 69 % at 6 months [51] to >90 % at 1 year [36], even with large and giant aneurysms. The PITA trial (Pipeline for the Intracranial Treatment of Aneurysms) included 31 aneurysms, nine were large and two were GIA’s. Follow-up complete aneurysm occlusion was observed in 28 of 30 (93.3 %) patients, and residual aneurysm filling was noted in two (6.7 %) [52]. Some users have used a coil-assisted flow-diverter technique, whereby coils are placed into the aneurysm to promote better aneurysm sac occlusion, particularly for large or acutely ruptured aneurysms. This has not been found to be associated with increased aneurysm occlusion rate [50]. Lubicz et al. advocates the use of additional coiling only in aneurysms with high risk of rupture or to restrict the PED coverage to a single device in order to minimize the risk of side branch occlusion [51]. If there is residual aneurysm after placing the PED, some interventionalists will place a second stent across the first one to further increase the metal coverage of the aneurysm neck.
The main limitation of the PED is the potential latency period before aneurysm thrombosis takes place; this is particularly challenging for ruptured aneurysms [34]. Complications of the PED include in-stent thrombosis (6 %), in-stent stenosis (1 %), intracranial hemorrhage (3.8 %), and perforator vessel occlusion [37]. Szikora reported a 1.75 % rate of severe hemorrhagic complications (mostly delayed ipsilateral parenchymal or subarachnoid hemorrhage) after using the PED, resulting in a 0.75 % permanent morbidity rate and a 1 % mortality rate [53]. In PITA, two patients had periprocedural stroke, and no other neurologic deterioration was observed in any of the patients at discharge [52].
Siddiqui et al. [54] recently reported their experience with flow-diverting stents in the treatment of large or giant intracranial vertebrobasilar aneurysms in seven patients. Pipeline devices were placed in six patients and the Silk Device (Balt Extrusion; Montmorency, France) in one patient. Four of seven patients treated with the PED died on follow-up, the other three had mRS scores of 5 (severe disability), 1, and 0. The deaths resulted from aneurysmal rupture in two patients and poor neurological status related to presenting brainstem infarcts and subsequent withdrawal of care in the other two patients. This report has raised questions about the safety of flow diversion in the posterior circulation.
Case study 5: A 51-year-old woman presented with new-onset cranial nerve III palsy. MRI imaging revealed a giant right cavernous carotid artery aneurysm. The PED was selected to treat the aneurysm. The patient was started on 3 days of aspirin 325 mg daily and 5 days of clopidogrel 75 mg daily. Platelet aggregometry was performed to ensure adequate platelet inhibition.
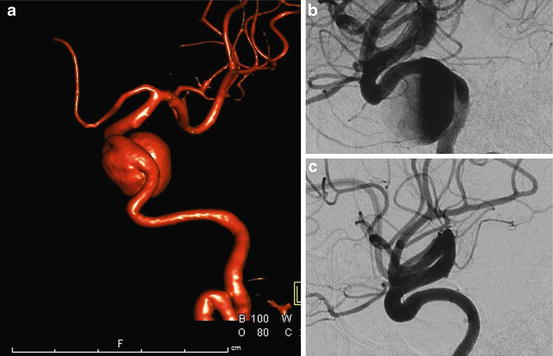
A 6-F sheath was placed in the right common femoral artery. Unfractionated heparin was administered to achieve an ACT ≥ 250. A 5-F Simmons II Glidecatheter was navigated into the right internal carotid artery with the aid of a 0.035-in. Glidewire. The diagnostic catheter was then exchanged over a 0.035-in. Bentson wire (Boston Scientific; Natick, MA) for a 7-F Shuttle sheath that was positioned within the proximal right internal carotid artery. A Navien (Covidien; Irvine, CA) 0.058-in. intracranial support catheter, a Marksman (Covidien; Irvine, CA) microcatheter, and a Synchro 14 microwire were navigated past the aneurysm under roadmap guidance (Fig. 10.5a). The microwire was removed and a 3.5 mm × 25 mm PED was deployed across the aneurysm neck creating stagnation of flow within the aneurysm (Fig. 10.5b). The 6-month follow-up angiography showed complete remodeling of the parent vessel with resolution of the patient’s cranial neuropathy (Fig. 10.5c).

Fig. 10.4
An example of vessel sacrifice to treat a partially thrombosed giant cavernous carotid artery aneurysm (a) with adequate collateral supply via the anterior communicating artery post-occlusion (b)

Fig. 10.5
A GIA of the left cavernous carotid artery (a) treated with PED leading to immediate intra-aneurysmal stasis (b) and 6-month complete vessel remodeling (c)
References
1.
2.
3.
Wiebers DO, Whisnant JP, Huston 3rd J, Meissner I, Brown Jr RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.PubMedCrossRef
4.
UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–82.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








