Figure 8.1. Multiple episodes of systemic hypoxia with ischemic brain. In 30% of cases, these episodes are related to systemic desaturation (lower Figure, SaO2 and arrows). Decrease in SjO2 (gray arrow) shows foreseen events. The answer in tissue oxigenation shows a a decrease of more than 20 mmHg after 21 hours. The starting “hyperemia” peak (SjO2 >85%) is related to psychometric agitation and should be read in relation to median arterial pressure.
8.2.1 Global Approach: Jugular Bulb Oxygen Saturation
Introducing a catheter into the jugular venous bulb provides access to cerebral venous blood and thus permits indirect measurement of brain metabolism and/or an estimate of the amount of oxygen bound to hemoglobin. This amount is expressed as a percentage rather than by the partial pressure. According to the saturation curve of hemoglobin and oxyhemoglobin at the venous end, a partial pressure of oxygen of 40 torr corresponds to a saturation of 75% [42]. The frequent occurrence of artefacts and the wide variability in venous drainage of the brain produce inconsistencies in the values measured and reduce the reliability of SjO2 measurements [43]; nonetheless, the knowledge derived from SjO2 measurement can be useful in neurocritical care. It is essential that the catheter be positioned (and maintained) in the jugular bulb, as placement in a more proximal position can contaminate it with extracerebral venous blood, making values inaccurate and non-representative of the cerebral condition. By applying the Fick principle to this monitoring technique, changes in cerebral blood flow (CBF) can be inferred, adding more information to support the treatment of TBI and other entities than would be available from ICP monitoring alone [44,45]. Such inferences are valid only when the rate of oxygen consumption and transport, the amount of dissolved oxygen in the blood, the hemoglobin level, and the degree of decoupling of the saturation curve of hemoglobin are all stable [42,46]. Under evolving conditions, these factors need to be considered when interpreting SjO2 – a step which is not always taken. The global cerebral oxygen status in healthy individuals reflects the balance between oxygen supply and demand and is dependent on cerebral blood flow and metabolism. The cerebral metabolic rate of oxygen (CMRO2), expressed as ml/100 g/min, is calculated by multiplying CBF by the oxygen content difference between arterial and venous jugular oxygen (∆AjO2). The equation is expressed as:
CMRO2 = CBF x ∆AjO2 or CMRO2 = CBF x (CaO2 – CjO2)
where: CaO2 = arterial oxygen content; CjO2 = jugular venous oxygen content; and ∆AjO2 = arteriovenous jugular difference of oxygen.
In normal conditions, when metabolism is coupled with flow, the CMRO2 will not change [47]; hence, ∆AjO2 is constant. The normal value is 6.3±1.2 vol. /100 ml of blood [47,48]. In pathological conditions, however, CBF regulation may be abnormal and the coupling between flow and metabolism partly lost. For example, in patients with TBI, CBF can be increased or decreased regardless of CMRO2 [48,49]. Assuming a normal arterial oxygen content, narrowing of the ∆AjO2 with a SjO2 >75% indicates that the CBF is too high (>50 ml/100 mg/min) in relation to metabolic requirements and suggests hyperemia. Hyperemic values would be considered when the ∆AjO2 is <4 ml/dl. Conversely, a wide ∆AjO2 >9 ml/dl or a low SjO2 <55% suggests that the CBF is too low to meet metabolic needs and is indicative of hypoperfusion [48].
Normal parameters | Hypoperfusion | Hyperemia | References |
∆AjO2 2.9-6.3±1.2 ml/dl or 1-3 µmol/ml | 6.8 ml/dl or 3 µmol/ml | <3 ml/dl or <3 µmol/ml | [48] |
CBF 42±12 ml/100 g/min | 23 ml/100 g/min | 53 ml/100gr/min | [48] |
SjO2 60-75% | <55% | 75% | [50,51] |
Lactate oxygen index=LOI AV∆/Aj∆O2 <0.8 | Lactate index ≥0.8 Ischemic-infarct pattern | Normal LOI | [40,54] |
Cerebral Oxygen Extraction=CEO 35% (24-42%) adults 26% (17-35%) infants | >35% | <26% | [51,52,53] |
Table 8.1. Normal and pathological oxygen values in TBI.
When regulatory mechanisms are intact, there is an inverse relationship between ∆AjO2 and CBF. When CBF falls, the brain extracts a greater amount of oxygen and ∆AjO2 increases. However, when oxygen extraction is already high and the compensatory mechanisms are exhausted, a further decrease in CBF causes a decrease in CMRO2. Therefore, in cerebral ischemia, the oxygen uptake is dependent on the input and the relationship between CBF and ∆AjO2 and it is also unpredictable. This situation is associated with increased lactic acid production and can be detected by calculating the lactate-oxygen index (AV∆L/Aj∆O2). A lactate-oxygen index ≥0.8 indicates the presence of ischemia [40,48,54].
In TBI patients, a ∆AjO2 <2.9 ml/dl corresponds to an average CBF of 53±18 ml/100 g/min, a ∆AjO2 between 2.9 ml/dl and 6.8 ml/dl corresponds to a CBF of around 42±12 ml/100 g/min, and a ∆AjO2 >6.8 ml/dl corresponds to a CBF of about 23 ml/100 g/min [48]. Although calculating ∆AjO2 provides detailed information, drawing blood involves a heavy workload, so continuous SjO2 monitoring is preferable.
In summary, the SjO2 can be influenced by many variables involved in global oxygen supply and consumption. It reflects the balance between cerebral oxygen supply and metabolic rate when oxyhemoglobin saturation, hemoglobin concentration and the hemoglobin dissociation curve remain constant. A decrease in SjO2 reflects a decrease in oxygen supply to the brain or increased metabolic activity. Any disturbance that increases the demand or decreases the oxygen supply may decrease SjO2. Likewise, a disorder that decreases CMRO2 or increases the oxygen supply can increase the SjO2.
For a better understanding of the relationship between CBF and CMRO2, we may consider cerebral hyperemia as being similar to the hyperdynamic state in sepsis, in which cardiac output is high with a narrow ∆AjO2, with poor oxygen consumption and arteriovenous shunting [55]. In contrast, prolonged ∆AjO2 with SjO2 <65%, corresponds to a hypodynamic state, with pump failure and extensive extraction [56]. Clearly, this is only a comparison, because the vascular autoregulation in the brain is very different from that in other parts of the body (Figure 8.2).
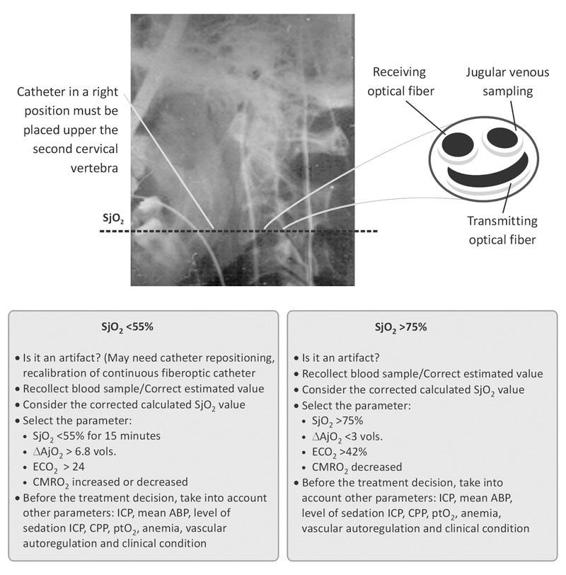
Figure 8.2. Key points in SjO2 monitoring. Main questions and management flow chart.
8.2.2 Practical Approach: Hyperemia with SjO2 >75%
To properly interpret SjO2 measurements, it is necessary to consider other variables of multimodality monitoring, and ICP monitoring in particular. A SjO2 >75 % (∆AjO2 <3 vols.) which coincides with an increase in ICP should be initially treated with rapid correction of hyperthermia, if present, and a moderate degree of hyperventilation [57,58] (PaCO2 30-35 mmHg) as the main agent. Increased sedation may be appropriate if levels are considered insufficient to reduce agitation [59,60]. Prior to initiating treatment, it is necessary to:
- Check for possible jugular catheter malposition.
- Check the calibration: ensure that the value estimated by the fiberoptic catheter does not vary by more than 4% compared to the saturation measured by the co-oximeter, while slowly withdrawing blood from the catheter tip [43].
It is recommended to calibrate the SjO2 monitor with the co-oximeter every 6 hours when changes in SjO2 occur and prior to initiating therapeutic changes. Rotation movements of the neck or cervical extension facilitate the loss of the optical signal, since the fiberoptic catheter then touches the walls of the vein (Figure 8.3).
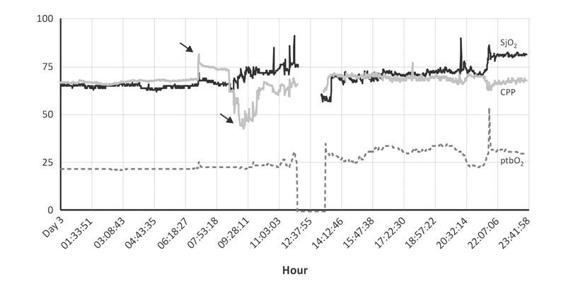
Figure 8.3. SjO2 monitoring and common artefacts. During the bath, SjO2 (previously increased with CPP, first arrow) decreased by 45 mmHg (second arrow). No consistent variation in CCP in relation to ptbO2 was observed. With the fast and inexplicable fall of SjO2, blood was extracted and SjO2 changed. Between 6.18 and 7.53 hrs, with the aspiration of secretions, SjO2 increased, suggesting hyperemia. The period of monitor disconnection shows ptbO2 reliability and recalibration of SjO2 (registered between 12.37 and 14.12 hrs).
Clots can also alter the light transmission of the optical fibre [43]. Table 8.2 shows important factors for the treatment decisions of patients with SjO2 >75 % and SjO2 <55 % by means of other elements of multimodality monitoring.
Factors favoring SjO2>75% | Factors favoring SjO2 <55% |
|
|
Table 8.2. Factors decisions in Hyperemia and Hypoperfusion interpreted by SjO2 in TBI.
8.2.3 Theoretical Approach: Hyperemia With SjO2 >75%
Hyperemia is common in the immediate postoperative period after craniotomy [61]. The CBF in the area adjacent to a craniotomy may increase which – particularly in combination with an increase in blood pressure – can lead to vasogenic edema. When the brain is decompressed following the removal of a space-occupying lesion [5], the ICP decreases, sometimes leading to an abrupt increase in perfusion. This type of hyperemia can be indirectly detected with SjO2 (high values), but SjO2 may later decrease when the metabolic reserve is exhausted and CBF has become insufficient [62,63]. Blood pressure control becomes critical at this moment in order to limit the development of vasogenic edema. Because agitation and pain may cause increased blood pressure, it is better to initiate appropriate levels of sedation and analgesia before treating hypertension with antihypertensive drugs such as nifedipine or nicardipine [64-66] (Figure 8.4).
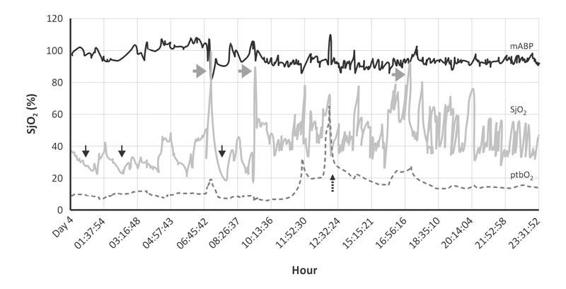
Figure 8.4. Male, 15 years old, with GCS 8, CT Scan with diffuse small hemorrhagic points after traffic accident. Several events of hyperemia (gray arrows) and hypoperfusion (black arrows) in TBI patient, not fully sedated and febrile. The repercussion in ptbO2 was evident; ptbO2 decreased by 5 mmHg with an associated reaction in SjO2. Dotted arrow shows fest FiO2 100%. The blood pressure had an increasing pattern trend. Hypoperfusion (black arrows).
in 30% of patients with severe TBI, nursing procedures such as tracheal suctioning in the first 36 hours posttrauma increased blood pressure, ICP and SjO2. ICP remained elevated for 11±6.1 minutes by 5 mmHg or more (mean increase of 16.6±10.2 mmHg) and SjO2 for 15.0±12.4 minutes, rising from 62±10 to 77±10%. The blood pressure increased for at least 10 minutes (average, 16.0±8.0 minutes) during and after 94 of the 120 aspirations, resulting in an increase in cerebral perfusion pressure (CPP) from 80.7±11.9 to 97.4±18 mmHg. The sustained rise in SjO2, with increased perfusion pressure during and after suctioning, suggests disturbed vascular autoregulation [67]. The impact that such events may have on outcome is uncertain. Sometimes, autoregulation can partially self-regulate; increasing the blood pressure with vasopressors may reduce ICP and improve the SjO2.
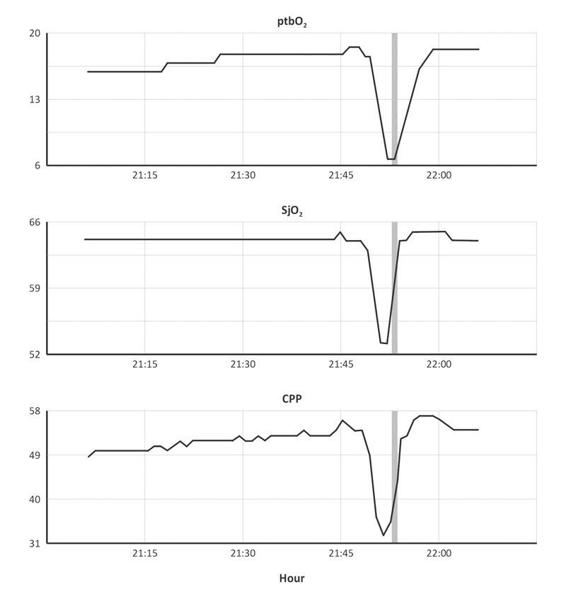
Figure 8.5. Evident disfunction in cerebrovascular autoregulation. The cerebrovascular autoregulation is dependent from the cerebral pressure perfusion (CPP), especially in cases with vasopressors. In this case, CPP was really low, below the normal range. SjO2 and ptbO2 decreased at the same time of CPP (21.45 h).
It should be remembered, however, that a single determination of SjO2 >75% by itself does not imply a correct CPP nor does it define the prognosis [68].
Much can be learned about the incidence of hyperemia in TBI as determined by SjO2 from studies by Robertson et al. [69]. A total of 2799 samples were taken from 450 patients in the first 5 days after trauma. Normal values were found in 342 patients (76%), high values (>75%) in 86 (19.1%), and low values (<55%) in 22 (4.9%) (Figure 8.6).
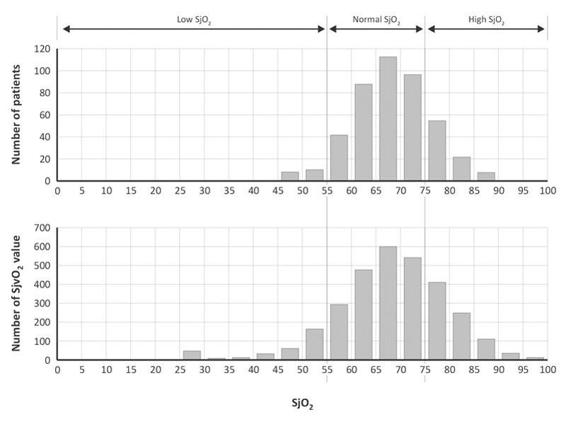
Figure 8.6. Graphs showing the distribution of the SjO2 values among the 450 patients (upper) and distribution of the 2799 SjO2 individual measurements (lower), with division into three groups with low, normal, and high SjO2 values.
The distribution of SjO2 values was found to change over time. On days 1 to 5 posttrauma, about 30% of patients had a SjO2 >75%. No clear correlation was found between SjO2 and CBF, however [42,69]. Low CBF values occurred at high values of saturation and high CBF values at lower SjO2 values. Patients with an average SjO2 >75% had a poorer outcome, most likely secondary to a depressed metabolic rate rather than due to hyperemic CBF. In this study, SjO2 >75% was not clearly associated with hyperemia, although some authors consider it a major cause of intracranial hypertension [70,71].
Also, a wide range of CPP values was found at elevated SjO2 values, indicating that hyperemia cannot be accurately identified by measuring SjO2. Regional CBF can be high in some parts of the brain but low in others [49,72]. These findings stand in contrast to those of other studies, where a closer association between SjO2 and hyperemia was found in smaller groups of patients. What we can conclude is that hyperventilation or other treatments to induce vasoconstriction should be initiated to decrease suspected hyperemia if patients are adequately monitored [59,60]. This makes a strong case for multimodality monitoring as a basis for treatment decisions [73].
In 1982, Chan [49] investigated the association between CPP and SjO2 in 41 TBI patients by means of continuous transcranial Doppler measurement to determine the optimal CPP level. At a given SjO2 level, the average velocity in the middle cerebral artery was measured and the pulsatility index calculated. The pulsatility index is defined as:
Pulsatility index: systolic velocity – diastolic velocity/mean velocity
(normal value = 0.9±0.2 in 20 subjects)
Regression analysis demonstrated that a decreased CPP, due either to an increase in ICP or a drop in blood pressure, increased the pulsatility index and reduced the SjO2. Decreases in SjO2 occurred more frequently at CPP levels <70 mmHg. At CPP levels >70 mmHg, however, there was no correlation between the pulsatility index and SjO2. Transcranial Doppler studies and calculation of the pulsatility index may therefore provide support in the search for an optimal CPP at a given level of SjO2.
8.3 Hyperemia and Arterial pCO2
Vascular tonus is strongly dependent on arterial pCO2. Increasing pCO2 and hypercarbia (PaCO2 >45 mmHg) causes vasodilatation, with an increase in cerebral blood volume and an increase in CBF. For each mmHg increase in PaCO2, CBF increases by 3 to 4% and cerebral blood volume by 0.72±0.42 [74,75]. Changes in arterial CO2 concentration affect its influence not only on vascular tone through changes in pH, but also through altered concentrations of adenosine and nitric oxide mediators which are considered mediators of vascular tonus [76]. An increase in PaCO2 raises ICP because increases in blood volume lower the volume-pressure index (see explanation in Annex 1) and spatial compensation capability.
The reduced reserve capacity to deal with increased volume at higher levels of arterial pCO2 has direct relevance for clinical management [59,60]. In situations of increased arterial pCO2, relatively minor and otherwise innocent increases in cerebral blood volume, which may be caused by the use of positive end-expiratory pressure (PEEP), venous obstruction, flexing of the neck or recruitment maneuvers, may lead to an unacceptable increase in ICP [77,80]. Therefore, caution is recommended in the use of permissive hypercapnia and lung-protective maneuvers in these situations [81].
Pulmonary problems caused by aspiration, contusion, rib fractures and atelectasis are common in TBI patients. Adjuvant CO2 retention with a decreased vital capacity and an increase in dead space may cause secondary brain damage due to increased cerebral blood volume, ICP and SjO2. Acute elevations of arterial pCO2 which increase ICP can generally best be managed by short-duration manual ventilation with an Ambu bag. Monitoring the effect of such maneuvers is important, as excessive vasoconstriction may result in cerebral ischemic events (Figure 8.7).
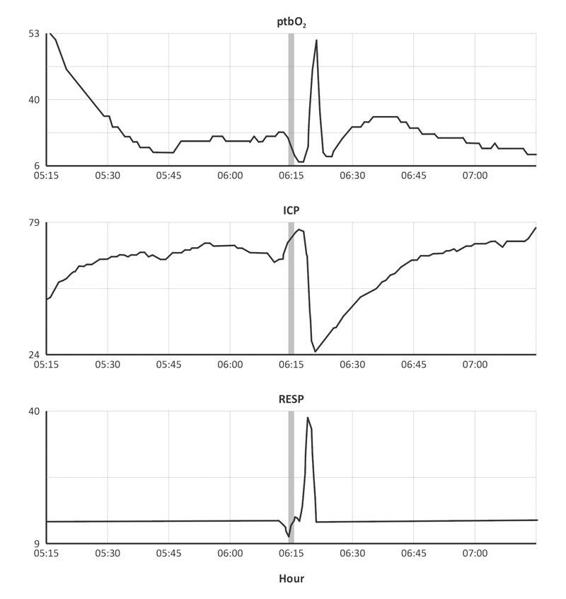
Figure 8.7. Refractary ICP and hyperventilation maneuver. The Ambu bag maneuver decreased ICP from 79 to 24 mmHg (6.15 hour). The initial elevation of mean ICP (5.15 h) was associated with a dropping trend of ptbO2. Later ptbO2 reached 53 mmHg with hyperoxia FiO2 100%. With Ambu bag maneuver (RESP=40) ICP decreased for around 15 minutes (6.15 h), and ptbO2 had a “superficial” improving pattern.
There are reports of the use of airway pressure release ventilation (APRV) mode to protect against elevation of ICP and to promote the elimination of pCO2 [82,83]. Importantly, however, any process that increases the alveolar pressure will also increase the pressure in the pulmonary artery and veins, thus carrying some risk for hypercarbia and suboptimal perfusion due to increased alveolar dead space. Much uncertainty exists on the effects of alveolar plateau pressure levels and the consequent production of CO2 during recruitment maneuvers in ICP.
An artificial maneuver that may mimic hyperemia is the administration of 100% oxygen prior to suctioning procedures. In hyperoxia, SjO2 increases markedly, thus potentially minimizing the risk of temporal dysperfusion and cerebral hypoxia in compromized patients [84,85] (Figure 8.8).
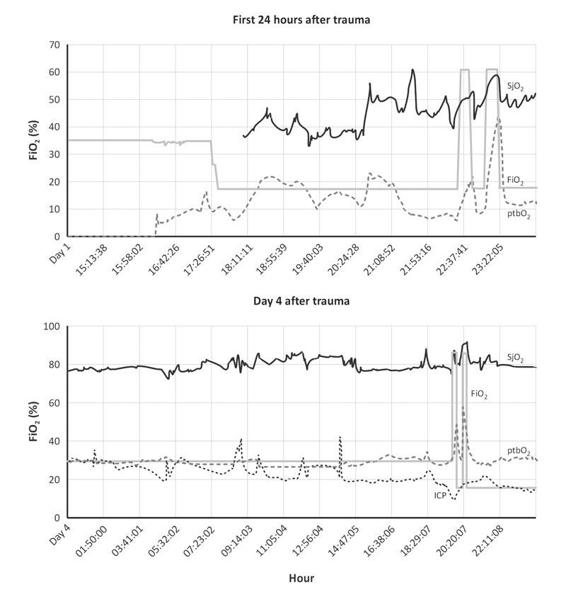
Figure 8.8. Ischemic pattern and hyperoxia response. On the initial 24 hours after trauma the oxygen parameters SjO2 an ptbO2 shows lower values (SjO2= 55 % and ptbO2= 15mmHg). From 18.11 until 22.59 abnormal lower values were observed. With hyperoxia 100 % FiO2 both parameters increased: ptbO2 reached 40 mmHg and SjO2 60%. The main suggestion for treatment will be to find the main cause of deterioration. Ischemic pattern an hyperoxia response. On day 4 after trauma the ischemic pattern improves. ICP decreased progressively, ptbO2 and SjO2 had a better trend. With FiO2 100 % both parameters increased, ptbO2 reached 50 mmHg and SjO2 90%, (20.20 hrs), with a hyperemia response. The appearance of hyperemia was unmasked by FiO2 % 100 and better condition of this subdural hematoma, GCS 4, with isocoric and reactive pupils.
The response is immediate and oxygenation can be maintained for the duration of suctioning procedures. The use of SjO2 monitoring can therefore provide opportunities for quantifying the quality of nursing activities and permits the verification of adequate pre-oxygenation prior to suctioning procedures. Hyperoxemia with delivery of 100% FiO2 as a therapeutic maneuver has been proposed by various groups [84-87]. Increasing PaO2 has been shown to decrease tissue lactate as measured by microdialysis [88]. Its effects on metabolism appear promising, but possible oxygen toxicity should be considered [89].
8.4 Approaches to Treatment
For treating injuries from a pathophysiologic perspective, Cruz proposed three stages of altered oxygen consumption in relation to CBF and SjO2:
- When a decrease in neuronal activity, aerobic metabolism and CO2 production leads to a proportionate decrease in CBF, cerebral oxygen extraction remains normal. This indicates a good balance between flow and metabolism.
- When the CBF is low in relation to oxygen consumption, cerebral oxygen extraction increases to compensate for the low flow state, and this condition represents oligaemic cerebral hypoxia.
- When the CBF is relatively high in relation to oxygen consumption (defective metabolic autoregulation), cerebral oxygen extraction decreases. This condition represents relative hyperperfusion or “luxury perfusion” [90].
Treatment for elevated ICP can be differentiated according to each of the above scenarios. In hyperemic flow with decreased cerebral extraction, Cruz proposed the concept of “optimized hyperventilation”, in which hyperventilation is targeted to decrease PaCO2 to levels <25 mmHg or even <20 mmHg [52]. This approach remains controversial because of the high risk of cerebral ischemia in the acute phase after injury and because of the heterogeneity of CBF in different areas of the brain.
The concept of optimized hyperventilation assumes that vasodilatation and CBF are homogeneous; however, this is not necessarily the case. Caution is therefore recommended when using optimized hyperventilation under the supervision of SjO2 and it should be used with moderation [59,60].
By contrast, optimized hyperventilation is inappropriate when the ICP is >20 mmHg and associated with increased or normal brain oxygen extraction. Cruz proposed the use of manitol, whilst other other authors recommended either hypertonic solution or barbiturate coma [52,91].
Summary of Hyperemia as Indicated by SjO2 >75%
- High SjO2 values are primarily caused by a low cerebral metabolic rate.
- Hyperemia as indicated by SjO2 >75% occurs predominantly after the first 48 hours.
- Although hyperemia may be a cause of intracranial hypertension, high SjO2 values are also found in patients with normal ICP.
- The pathophysiologic mechanisms underlying hyperemia are potentially reversible through respiratory modifications and metabolic intervention.
- Hyperemia as indicated by a high SjO2 can be associated with severe disturbances in cerebral autoregulation.
- Prior to initiating treatment for high SjO2 values, calibration errors should be excluded.
8.4.1 Practical Approach: Hypoperfusion due to SjO2 <55-60%
Low SjO2 values, which reflect increased oxygen extraction in relation to flow, are indicative of an increased risk of ischemia [50]. The therapeutic approaches in these patients should be aimed to increase oxygen delivery. In patients with raised ICP and a SjO2 <55%, we recommend correcting hemoglobin levels as a first step if these are low. Whilst a restricted approach to transfusion is currently recommended in general trauma following the demonstration of a lower mortality, it should also be remembered that brain trauma is a different situation [93,94].
Cerebral ischemia is particularly frequent in TBI patients with anemia and cerebral hypoperfusion. Much evidence exists that cerebral ischemia is a commonly encountered problem after TBI, particularly in the first 24 hours [50]. In this period, low CBF values and low values of jugular venous saturation (<65%) have been shown to occur frequently. Short-duration hypotensive episodes often cause SjO2 to fall to values <65%, illustrating the deleterious effect of low blood pressure on cerebral oxygenation[95]. In their histopathology studies, Teasdale et al. demonstrated the presence of cerebral ischemia in over 90% of cases [96].
More recently, magnetic resonance imaging (MRI) studies have shown that hypotension after TBI is associated with neuronal loss in the thalamic region [97, 98]. In a study by Robertson et al. [50,95], hypotension was the leading cause of episodes of jugular venous desaturation. Insufficient cerebral oxygenation and insufficient blood flow due to hypotensive insults are an important cause of secondary brain damage after TBI.
The effects of such secondary insults may be greatest in patients with intracranial hematoma. Many patients with cerebral contusion or intracranial hematoma show secondary deterioration and often present with the clinical syndrome of “talk and deteriorate” [99]. This illustrates that outcome is not only determined by the primary lesion and serves to highlight the possibly devastating influence secondary brain damage can have.
Reports from the Manchester Emergency Services highlighted poorer outcome in moderate TBI patients with associated systemic injuries [100]. The priority of preventing and treating cerebral ischemia is to target appropriate levels of blood pressure and arterial oxygenation. Critical decreases in SjO2 <50% for 10 minutes or longer are absolute indications for instituting vigorous therapy [50]. Early therapeutic interventions aimed to avert such critical episodes are strongly recommended.
Prevention and treatment of cerebral hypoperfusion requires adequate fluid resuscitation and the combined use of vasopressors. Various authors state a preference for the use of norepinephrine [101-103], as the drug is more selective and its effects more constant (see section on regional monitoring). Rosner devised eight rules to identify the best point of perfusion level based on multimodality monitoring [104] (Table 8.3).
|
Table 8.3. Rosner’s rules to identify the best point of perfusion level based on multimodality monitoring.
The optimal CPP level can vary between individual patients. Several studies suggest aiming at values of 60 to 70 mmHg [104,105] and international guidelines recommend maintaining CPP >60 mmHg [59,60], although lower levels may be considered acceptable in selected patients. When taking SjO2 into account, it should be remembered that the detection of desaturation events requires continuous monitoring as short-duration episodes, which may be very harmful and are not detected by intermittent monitoring [106]. Desaturation events can occur in up to 40% of TBI patients.
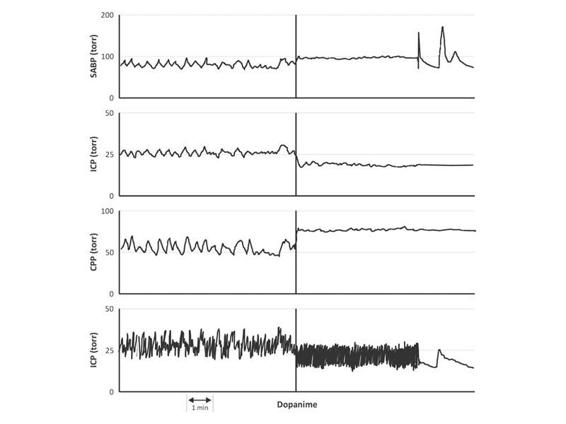
Figure 8.9. The vasopressor effect of dopamine decreases ICP. Upper panels show sistolic arterial blood pressure (SABP), ICP, and CPP. The insufficient systolic blood pressure around 90 mmHg was not the better combination with ICP of 35 mmHg. Vasopressor effort decreased ICP to 20-25 mmHg.
In patients with low SjO2 values, therapeutic modalities that may increase vasoconstriction, such as hyperventilation, should be avoided. Also, it is advisable to frequently reassess the acid-base balance, specifically with regard to paCO2 and respiratory alkalosis, by means of regular blood gas analysis and continuous monitoring of end-tidal CO2.
Following the correction and optimization of systemic parameters (anemia, hyperthermia, arterial pCO2 and arterial blood pressure), every effort should be made to determine the cause of desaturation in order to target therapy more appropriately. If low blood pressure is the problem, this should be corrected; if a low CPP has resulted from increased ICP, then the management should be primarily directed at treating the raised ICP.
8.4.2 Theoretical Approach: Avoiding Risks of Hyperventilation in Patients with Hypoperfusion (SjO2 <55%)
Lowering arterial pCO2 by hyperventilation is a recognized and effective approach to reduce elevated ICP [107]. The mechanism of action is by reduction of cerebral blood volume owing to vasoconstriction. Experimental studies using a cranial window have clearly confirmed that vasoconstriction of the pial arteries results from hyperventilation [108]. Studies using electroencephalography (EEG), however, showed that, whilst decreasing ICP, hyperventilation also produces flattening of wave form activity on the EEG [109].
Clinical evidence also exists that hyperventilation may compromise cerebral blood flow and oxygenation. In the first 24 hours after injury, global CBF is reduced in 30-70% of patients with severe TBI. In such patients the risk of cerebral ischemia on instituting hyperventilation may be particularly high [110]. An early work by Obrist [111] demonstrated that hyperventilation to an arterial pCO2 of 23.2±2.8 mmHg reduced CBF to 18.6±4.4 ml/100 g/min and increased arterial venous content differences by 10.5±0.7 vols. %.
Subsequent studies have shown that hyperventilation is the second most common cause of desaturation following a hypotensive episode [50,51]. Deliberate or inadvertent hyperventilation episodes may occur during the entire clinical course, extending from the injury site to the emergency room and the intensive care unit. The risk of hyperventilation is particularly high during transport [112] and is not always recognized.
In a descriptive study investigating the effects of hyperventilation in 21 patients in Mexico City, we found that in 82.6% of 97 samples the arterial pCO2 was <30 mmHg and SjO2 <65% in 45% of the samples [113]. These studies were performed using intermittent SjO2 monitoring with a co-oximeter, and the real incidence of jugular desaturation was probably underestimated. A strong case can be made for implementing further education and awareness to prevent and correct the “routine” use of hyperventilation, and this approach should be supported by motivation and training.
Level II guideline recommendations advise against hyperventilation in patients without elevated ICP. This recommendation is based on a relatively small number of patients, but the real impact of hyperventilation on outcome after TBI is uncertain. We suggest that hyperventilation may be definitely deleterious in some patients, but – if monitored closely – it may be beneficial in others.
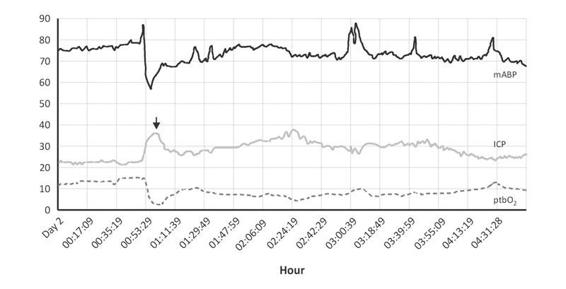
Figure 8.10. Effect of adrenergic activation in oxygen pressure tissue and ICP. A male, 15 years old, was injured by a car while riding a bicycle. The initial GCS was 8, EMV: 3, 3,2. He had a right tibia fracture and fractures of 3th and 4th left ribs, with compressed perimesecephalic cisterns in the CT scan. The ICP increased (arrow) until 35 mmHg during the bone fixation procedure, for insufficient anesthesia, ptbO2 decreased (00.53 hour). The mABP rose until 90 mmHg, later decreased 60 mmHg. Probably ptbO2 decreased for secondary hypovolemia associated with ICP elevation, beside initial MABP around 70 mmHg.
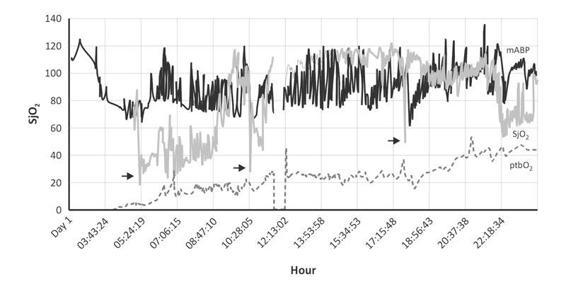
Figure 8.11. Initial correction of hypoperfusion and late hyperemia in SjO2. 58-year-old male, GCS = 6, 3-meter fall. On the admission his pupils were reactive. The CT scan showed diffuse hemorrhagic points with left parietal contusion and a middle line deviation of no more than 5 mm. Apparently the injuries were not really severe, it seemed that ICP was below 20 mmHg on the initial 24 hours after trauma. However, from 3.34 hours until 9.30 hours the oxygenation parameters showed marked hypoperfusion. Beside, mean arterial blood pressure (mABP) was higher than 60 mmHg. Later, SjO2 showed hyperemia (SjO2 >75%) not correlated with normal ICP. The mean ICP was below 20 mmHg. During this stage the mABP was 80-120 mmHg. The main cause of this vascular autoregulation was probably a higher arterial blood pressure. Three months after the trauma the GOS was 4. Arrows: fiberoptic SjO2 calibration.
There are well-documented risks associated with the use of hyperventilation to decrease CBF, to decrease brain oxygen tension, and to cause jugular desaturation [50,59,60]. More recent studies using positron emission tomography (PET) have shown that CMRO2 is unaffected by moderate hyperventilation [114]. Microdialysis studies, however, have shown that short-duration hyperventilation in the first 24 hours after injury can increase brain lactate and decrease cerebral extraction of oxygen [115]. Uncertainty exists about which patients may and which will not benefit from hyperventilation. Caution in the use of hyperventilation is advisable. The following recommendations can be made:
- Avoid hyperventilation in the first 24 hours after trauma and during transport. When applied, use hyperventilation only for short periods [59,60].
- Do not exceed a moderate level of hyperventilation (paCO2 30-35 mmHg) [59,60].
- Closely monitor and assess the end-tidal CO2 and/or arterial blood gasses at least every 6 hours.
These considerations illustrate that an understanding of the underlying pathophysiology in TBI patients is complex and that treatment approaches may carry both benefits and risks. Current insights into pathophysiology and results from multimodality monitoring strongly support the concept of a more individualized approach, instead of standardized protocols, in managing TBI patients.
Applying personalized approaches requires a full understanding of the ongoing pathophysiology. In the absence of dedicated multimodality monitoring, we do not know whether the patients may be in an oligaemic or a hyperperfusion state. And although important, ICP monitoring only provides information on a single parameter. It should be remembered that prophylactic hyperventilation will not prevent increases in ICP [59]and that hyperventilation may decrease cerebral oxygenation, as measured by brain tissue oxygen pressure, in patients with normal and those with elevated ICP [59,60].
Further insight into the pathophysiology of individual patients can be gained from measuring both global (SjO2) and regional (brain tissue oxygen pressure) cerebral oxygen parameters. Examples of different treatment approaches are summarized in the figures (Figures 8.10-8.12).
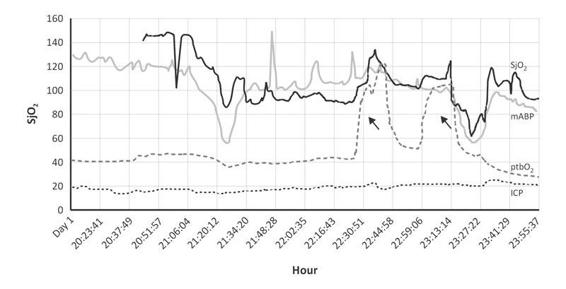
Figure 8.12. Hypotension and hypoperfusion in a hypertensive patient with TBI. In TBI the main secondary injury factor associated with a poor outcome is hypotension. In this patient, GCS 7, with isocoric pupils (negative/positive), the mean arterial blood pressure decreased from 120 to 50 mmHg (20.00 to 21.20 hours) with associated effect in SjO2 and ptbO2. Apparently the main effect was observed in SjO2 which persisted until the first maneuver 100 % FiO2 (first arrow); SjO2 increased until 80%, together with mean arterial blood pressure higher than 100 mmHg (22.44 hour). These hemodynamic changes indicate a severe affection. However, the ICP was normal. In chronic hypertension the key point for BP must be interpreted in relation with ICP, in order to test the vascular autoregulation limit. In the first initial 24 hour after trauma, sudden changes in hypotension and hypertension induce severe edema. The sudden hemodynamic change greatly increased ICP. On day 3, ICP reached 35 mmHg. Three months after trauma, GOS was 3 points.
These examples are not evidence-based but rather represent the consensus of experts experienced in TBI treatment and with a history of participation in clinical trials and research.
8.4.3 Regional Approach: Brain Tissue Oxygen Pressure
The regional approach relies on brain tissue oxygen monitoring, commonly abbreviated as ptbO2, ptiO2, or PbrO2. In this section we shall use the abbreviation ptbO2 (partial pressure of brain oxygen tension).
8.4.4 Development of a Clark-type Electrode for Measuring Brain Tissue Oxygenation
The technology for ptbO2 measurement originated from studies on photosynthesis. In 1938, Blinks and Skow used a platinum electrode as a cathode for measuring pCO2 in plant tissue [116]. Brink, Davies and Bronk attempted this technique in human tissue, but “the metal poisoned the blood” [117]. This report prompted Clark (Figure 8.13) to cover the cathode and anode with polyethylene to “prevent intoxication” [118].
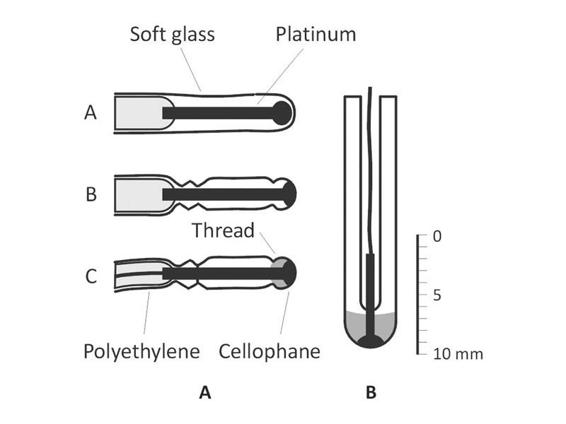
Figure 8.13. Catheter components: A) Catheter covered by cellophane. B) Catheter distal portion. A shiny platinum cathode covered with a layer of cellophane has been found to be suitable for the direct measurement of oxygen tension in whole blood by polarographic procedures. The cellophane covering prevents the undesirable effects of the red cells which interfere with oxygen tension measurements using a bare platinum electrode, and in addition nearly abolishes the “stirring effect”. A tiny cathode of this type (2 mm diameter and 8 mm long) mounted on a catheter can be passed into the major blood vessels and the chambers of the heart, for continuous recording of oxygen tension. Such cathodes are easily calibrated and are stable, reproducible and sensitive.
The polyethylene covering effectively reduced oxygen consumption by the electrode and facilitated the diffusion of environmental oxygen to the electrode. Since 1954 the Clark electrode has been used in the measurement of blood gases.
Although Clark attempted to monitor oxygen tension in the brain with this method, it was not until 1980 that Dr. W. Fleckenstein in Germany further refined the methodology for use in tissue measurements in experiments first on small animals and then on humans [119]. Measurement in both the brain parenchyma and the cerebrospinal fluid (CSF) showed higher levels of pO2 in the CSF compared to the parenchyma, with a much greater and more rapid influence of arterial pO2 on measured values. This was probably due to the rich vascularization of the choroid plexus where no blood-brain barrier is present. Reperfusion studies showed a much slower recovery of brain tissue pO2 following global ischemia than in the CSF. It was therefore concluded that brain tissue measurements would provide greater relevance to cerebral oxygenation in clinical use [120].
The basic principle behind the Clark-type electrode is that environmental oxygen diffuses through the polyethylene covering into a chamber containing a gold or platinum electrode immersed in an electrolyte solution. In the chamber, the oxygen is converted to OH– at the gold cathode to produce an electric potential that can be measured. Reduction of water with oxygen within this chamber is expressed according to the chemical reaction:
2H2O + O2 → 4OH–
thus providing 4 OH and generating the electric potential.
Initially, the electrodes were designed as fast-response systems to measure the electric potential very locally. This permitted study into the heterogeneity of cerebral pO2 and related cerebrovascular distribution, which could be expressed as ptbO2 histograms. The great heterogeneity of ptbO2 in brain tissue, however, made this approach difficult for clinical use.
For clinical application a catheter was designed with a larger measurement area and a slower response, thus averaging local heterogeneity and providing an average oxygen tension within a specific area of the brain from which oxygen could diffuse to the catheter. The ptbO2 thus measured can be considered as the average of values of thousands of capillaries surrounding the catheter, i.e., 200-10000 fields created by local diffusion. The ptbO2 reflects the balance between available oxygen flowing through the tissue microvasculature and local consumption. In other words, it is the partial pressure of oxygen in the extravascular and extracellular interstitial space. It does not measure the intracellular oxygen concentration in cell bodies. Normal values of ptbO2 measured with the Clark catheter technology are within the range of 20 to 40 mmHg, with an average of 25-27 mmHg at normal ICP and CPP levels [121,127,131] (Figure 8.14).
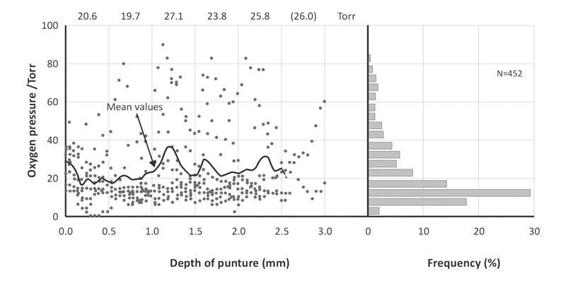
Figure 8.14. Heterogeneity and ptbO2 in experimental kidney (rat). Values of nine punctures in relation to the depth of the puncture. Significant heterogeneity for surface anesthesia. Low values coexist with values close to paO2 pressure.
8.4.5 Factors Influencing Brain Tissue Oxygen Tension
ptbO2 responds directly to oxygen tension in the blood and the number and diameter of capillaries per volume of measured tissue. More parameters are described in the following sections: FiO2, temperature, pressure perfusion, hemoglobin, paCO2, ICP, vascular autoregulation, etc. [122-131] (see Table 8.4 and Figure 8.15).
Local factors influencing ptbO2 | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



