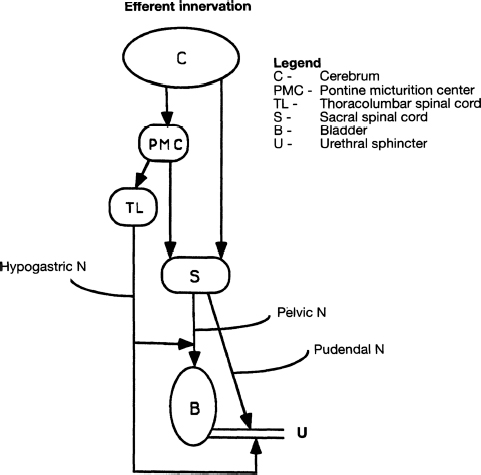
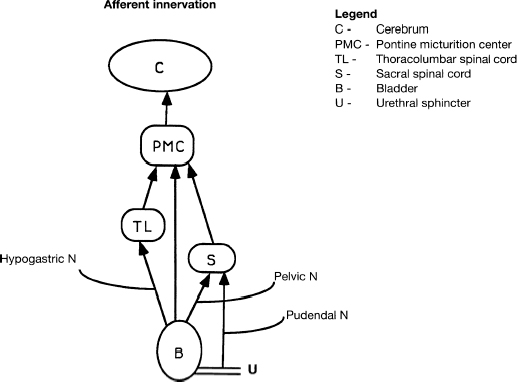
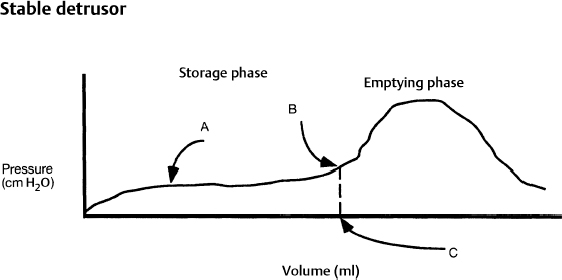
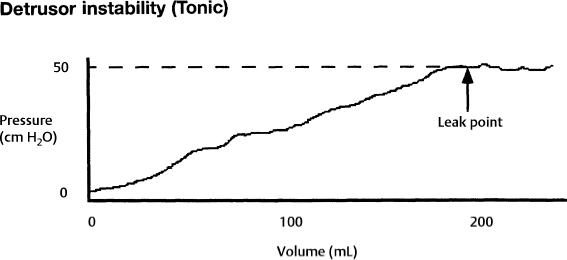
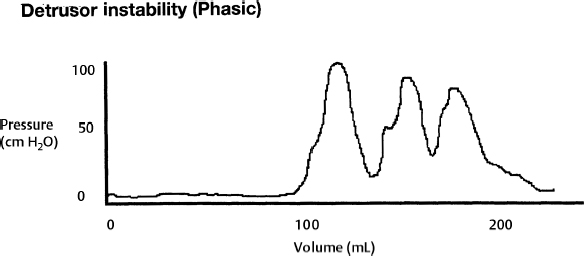
7 Urological Aspect of Tethered Cord Syndrome I: Lower Urinary Tract Dysfunction in Tethered Cord Syndrome H. Roger Hadley, Herbert C. Ruckle, Brian S. Yamada, and Gideon D. Richards Bladder dysfunction occurs in ∼40% of patients affected by tethered cord syndrome. It is the exclusive complaint in 4%.1 The most common urological symptom is incontinence; in some patients, it may be the earliest sign of the syndrome. Management of patients with voiding dysfunction secondary to tethered cord syndrome requires a basic understanding of bladder physiology and voiding mechanisms. This chapter provides an understanding of these principles. Bladder function has a twofold purpose: to store urine and to empty when socially acceptable. Despite the apparent simplicity of these two functions, storage and emptying of the bladder requires a complex interplay between the central nervous system, peripheral nervous system, urethra, and bladder. Normally, the bladder is a highly compliant structure accommodating continuous production of urine from the kidneys while maintaining low intravesical pressure. This low pressure protects the kidneys and ureters from hydronephrosis, urine reflux, and damage from transmitted pressure, and it permits urine storage without high urethral sphincter resistance. Continence of urine is dependent upon a competent urethral mechanism that can both maintain passive continence at rest and can almost instantaneously increase tone in response to the increases of intravesical pressure that occur throughout daily life. The process of bladder emptying is not a simple neuromuscular reflex; it requires a sophisticated coordination of urethral sphincter relaxation and bladder contraction. This process begins on command and continues to completion automatically, without further conscious neural input unless these pathways are voluntarily interrupted. The lower urinary tract receives efferent impulses from both the autonomic and somatic nervous systems via several pathways (Fig. 7.1). Sympathetic fibers exit the thoracolumbar spinal cord and descend via the superior hypogastric plexus and then bifurcate into the bilateral hypogastric nerves. The parasympathetic fibers emanate from the sacral spinal cord and become the pelvic nerves, which join the hypogastric nerves and form the pelvic plexus (inferior hypogastric plexus). Nerve fibers within this plexus reach their targets within the bladder and urethra, ensuring both sympathetic and parasympathetic innervation. The somatic efferent nerves, in contrast, travel through the corticospinal tracts to synapse on lower motor neurons in the pudendal nuclei of the sacral spinal cord. Motor impulses travel from these nuclei to their destination in the urethral sphincter muscle via the pudendal nerve. The pelvic, hypogastric, and pudendal nerves all route afferent impulses from the urethra and bladder to the central nervous system (Fig. 7.2). Upon entering the spinal cord, some nerve impulses travel along fibers destined for segmental reflex arcs with spinal nuclei, whereas others synapse upon nerves ascending within the spinal cord to convey the information to the supraspinal and conscious levels.2–5 Fig. 7.1 This diagram depicts efferent pathways from the cerebrum, pontine micturition center, and spinal cord centers to the detrusor and urethral sphincter mechanism. Fig. 7.2 This diagram depicts afferent pathways from the bladder and urethra to the spinal cord, pontine micturition center, and cerebrum. The concept of micturition reflex, in which coordinated detrusor muscle contraction and urethral sphincter relaxation are triggered by bladder distension, was introduced in 1900.6 Since that time, many models of reflex pathways have been proposed. The majority of the experimental evidence supports a supraspinal organization of the micturition reflex.7–9 The supraspinal region that serves to organize this reflex is the pontine mesencephalic micturition center (PMC).2,3,10 Conscious neurological control of the detrusor and urinary sphincters is provided by discrete cortical regions and is mediated via the PMC and directly through corticospinal pathways to motor nuclei in the sacral spinal cord. Neural connections of these pathways with pathways involving the midbrain, cerebellum, and other centers have been identified, but their functional significance is not yet clear.10,11 Current understanding divides the micturition cycle into two functional phases: the phase of bladder storage and the phase of micturition. The viscoelastic properties of the bladder wall afford high compliance during filling and storage, thus allowing low-pressure filling without neural input.12 After reaching a threshold volume, afferent impulses travel through the pelvic nerves up the spinal cord to the PCM and higher centers. This orchestrates the sympathetic efferent discharge from the hypogastric nerves to activate α-adrenergic receptors contracting the smooth muscle of the urethral sphincter and to inhibit parasympathetic perivesical ganglia via β receptors, thereby decreasing bladder tone and contractility. This sympathetic activity is coupled with cortical inhibition of the PMC and stimulation of the striated sphincter via the pudendal nerve. Together these actions are responsible for the appropriately contracted, continent urethral sphincter muscle and detrusor muscle quiescence that characterize the storage phase of bladder function.4,5,10,13,14 The micturition phase of bladder function is triggered in normal urinary systems by the progressive distension of the bladder and increasing afferent activity through the hypogastric nerves and pelvic nerves that occur as the bladder fills. The impulses reach the PMC and cortical centers, which produces a conscious sensation of a full bladder and the need to void. The PMC coordinates the transition into the voiding phase. Decreased activity in the pudendal nerve results in a relaxation of the urethral sphincter muscle. Efferent sympathetic discharge also decreases, thereby relieving the inhibition on the parasympathetic ganglia and allowing the smooth muscle of the bladder neck to relax. An increase in sacral parasympathetic outflow causes a coordinated contraction of the detrusor, and voiding ensues. Voluntary interruption of voiding can occur when somatic efferent impulse from frontal cortex contracts the voluntary sphincter, which increases bladder outlet resistance, and the detrusor muscle contraction reflexively abates.4,5,8,10,14 Like most aspects of medicine, a careful history and physical exam are essential to diagnose and treat a neurogenic bladder. It is vital to investigate other potential neurological symptoms. The patient’s urinary symptoms must be placed within the framework of both the bladder’s micturition and storage functions. Furthermore, it is important to elicit from the patient a clear picture of the patient’s normal diurnal and nocturnal voiding patterns and habits. One must ask specifically about symptoms of nocturia, urgency, frequency, hesitancy, straining, sensation of fullness, sensation of complete emptying, dysuria, ability to interrupt or inhibit micturition, urinary incontinence, bowel habits, ability to achieve erection and ejaculation, paralysis, weakness, numbness, paresthesias, or seizures. The patient’s past medical history should be probed for any spinal, pelvic, or neurological problems, trauma, or surgery. The possibility of the patient’s use of medications that affect the autonomic pathways that mitigate bladder function (a agonists and antagonists, anticholinergics, β blockers, opioids, caffeine, and other methylxanthines) should be explored. The physical exam should include a thorough evaluation of neurological and urological systems. The anal wink and the bulbocavernosus reflex are particularly useful because they correspond to the S2–4 levels where the somatic and parasympathetic efferent and afferent pathways intersect with the spinal cord.13,15–17 Urodynamic testing has emerged as the quintessential evaluation to explicitly identify, document, and quantify the effects of neurological dysfunction on the urinary system. Urodynamic parameter improvement has become a quantifiable outcome that is used to gauge the effectiveness of treatment for tethered cord syndrome. The evaluation consists of some combination of cystometry, uroflometry, and electromyelography performed during the phases of filling and micturition, with the goal of reproducing and evaluating a patient’s typical voiding cycle. Cystometry is the measure of pressure changes in the bladder throughout the voiding cycle. A pressure transducer is placed transurethrally into the bladder to measure intravesical pressure (pves). Both the tone of the detrusor muscle and intraabdominal pressure combine to produce the measured pves. To determine the actual pressure of the detrusor another pressure transducer is introduced to measure abdominal pressure (pabd). This transducer is typically placed in the rectum. A subtraction of the abdominal pressure from the intravesicular pressure results in the pressure contribution of the detrusor (pdet) (i.e., pves – pabd = pdet). The relationship of pdet and the patient’s sensation of bladder filling with bladder volumes (Fig. 7.3) during the filling cystometrogram and voiding allows the measurement of bladder compliance, bladder contractility, and maximum cystometric capacity. The patient’s subjective report of the first sensation of filling, first desire to void, and strong desire to void during bladder filling are used to evaluate the patient’s sensation and characterize it as normal, reduced, increased, or absent. The compliance of the bladder is a quantification of the viscoelastic properties of the bladder wall. Compliance is equal to the change in volume divided by the change in pressure during filling and is expressed in mL/cm H2O. The normal bladder has a high compliance during passive filling and the pressure volume curve of the cystometrogram (CMG) is relatively flat until the cystometric capacity is approached. A bladder with decreased compliance (Fig. 7.4) will exhibit a filling CMG with a steady rise in the pressure-volume curve. Contractility refers to the ability of the detrusor to increase the pdet during detrusor contraction. Involuntary detrusor contractions can be demonstrated on the CMG (Fig. 7.5), and this is referred to as detrusor overactivity (formerly detrusor instability). Fig. 7.3 Normal cystometrogram depicting the relationship between detrusor muscle pressure and intravesical volume during the storage and emptying phases of voiding. A, first sensation of filling; B, sensation of maximum fullness; C, bladder capacity. Fig. 7.4 Tonic detrusor instability describes a poorly compliant bladder with steeply rising filling pressures at low bladder volumes. The leak point pressure is important in evaluating the risk of upper tract deterioration. If detrusor overactivity is present and can be attributed to a neurological cause, the term neurologic detrusor overactivity is used (formerly hyperreflexia). If the detrusor does not contract during the CMG it is referred to as an acontractile bladder, and if it can be attributed to a neurologic cause, the term bladder areflexia applies (Table 7.1). Uroflowmetry measures the volume of urine expelled per unit of time, typically mL/s. The urine flow is determined by a combination of the effects of detrusor pressure and urethral resistance during micturition. Maximum and average flow rates can be determined. The voiding pattern can be displayed graphically, which allows its characteristics to be evaluated (e.g., interrupted flow versus continuous flow). Flow can be reduced by poor detrusor tone or bladder outlet obstruction as in the case of detrusor-sphincter dyssynergia (DSD) (Fig. 7.6). Fig. 7.5 Phasic detrusor instability describes an overactive detrusor muscle with spontaneous or provoked involuntary detrusor muscle contractions. The external urethral sphincter is composed of skeletal muscle. Electromyography (EMG) employs a needle electrode to measure the bioelectrical activity of urethral skeletal muscle. Quiescent at rest, as more muscle cells are recruited into contraction, the EMG tracing demonstrates increasing electrical activity. In normal micturition, the external sphincter relaxes, and the activity on the EMG drops immediately before detrusor contraction begins and tone returns after the completion of voiding.
Principles of Normal Bladder Function
Patient Evaluation for Neurogenic Bladder Dysfunction
Urodynamic Testing
Cystometry
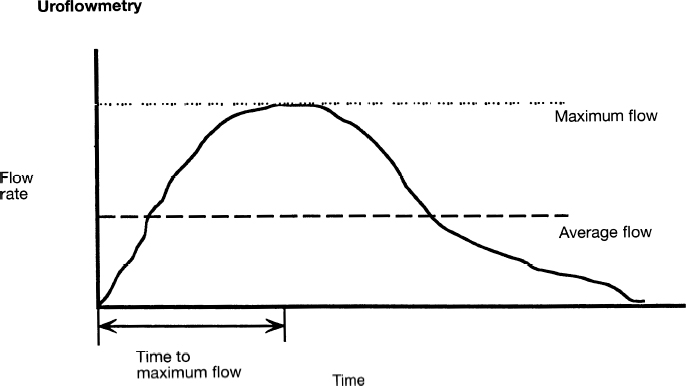
Uroflowmetry
Electromyography
| • Acontractile detrusor: Detrusor that cannot be demonstrated to contract during urodynamic evaluation |
| • Areflexia: A contractile detrusor due to an abnormality of neural control |
| • Detrusor-sphincter dyssynergia (DSD): Involuntary contraction of the external urinary sphincter muscle during an involuntary detrusor contraction |
| • Bladder compliance: The relationship between change in bladder volume and change in bladder pressure |
| • Postvoid residual: The volume of urine that remains in the bladder immediately after voiding |
| • Maximum cystometric capacity: The volume at which the patient feels he or she may no longer delay micturition and has a strong desire to void |
| • Urgency: A sudden compelling desire to void, which is difficult to defer |
| • Overactive bladder: Urgency with or without urge incontinence often with frequency and nocturia |
| • Detrusor overactivity: Involuntary detrusor contractions during the filling phase of urodynamics, which may be either provoked or spontaneous |
| • Neurogenic detrusor overactivity: Detrusor overactivity due to a neurological cause (formerly detrusor hyperreflexia) |
| • Clean intermittent catheterization (CIC): Aspirating or draining the urinary bladder with a catheter and subsequently removing the device. All performed with clean (not sterile) technique |
DSD describes the situation where the coordination of these events is lost and an involuntary external sphincter contraction persists during detrusor contraction, resulting in decreased flow, obstruction, and, often, increased intravesical pressure and kidney damage. DSD is a common finding in patients with neurological causes of bladder dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







