10 The use of steroids in the management of acute spinal cord injury (SCI) has been one of the most controversial topics over the last decade since the publication of the North American Spinal Cord Injury Study (NASCIS) clinical trials in the late 1990s and early 2000s. Currently, consensus opinion from the neurosurgery, spine, and emergency medicine communities in North America recommends steroids in the acute treatment of SCI only as an option with little clinical benefit.1–3 The consensus opinion illustrates the controversy surrounding the use of steroids, and a literature search on the subject easily uncovers the polarization of the medical community into staunch advocates of the clinical benefits of steroids in acute SCI and concerned clinicians who believe the complications from high-dose steroid therapy far outweigh the questionable clinical benefit. Steroid administration for SCI is partly based on observations of the potent inhibitory effects of high-dose steroids on vasogenic edema in neurological tumors.4 A wealth of small and large animal studies attempting to characterize the effect of steroids in SCI suggest that steroids have significant inhibitory effects not only on edema but also on inflammation, lipid peroxidation and a whole host of secondary effects that have been recently popularized in SCI models of secondary injury.5,6 However, it has become clear over the past few decades that the pathophysiology of edema in tumors is intrinsically different from edema seen in traumatic or hypoxic injury, and that the clinical use of high-dose steroids in traumatic neurological injuries is associated with significant risk of pneumonia, sepsis, and death.7–10 Advocates for the use of steroids in SCI are passionate about their cause. SCI is a devastating injury commonly affecting the young with high mortality and morbidity. The worldwide incidence of SCI is estimated to be 20 per 1 million people per year with a prevalence of 700 SCI cases per million.11,12 More than 55% of traumatic SCI occurs in people less than 30 years of age often as a result of sports or motor vehicle accidents.12 More than half of the injuries are localized to the cervical spine between C1 and T1, resulting in various grades of quadriplegia.13 Of those that survive to a tertiary care SCI institution, 4 to 17% die in hospital, whereas survivors go on to extended rehabilitation complicated with long-term sequelae such as spasticity, pressure sores, pneumonia, deep vein thrombosis, and renal calculi.11–13 Despite the revolutionary advances in molecular biology and bio-technology over the past 20 years, there is still an absence of effective pharmaceutical agents for ameliorating, modulating, or reversing SCI. Drugs such as steroids, with a suggestion of clinical benefit, are heralded as “the only option for a devastating condition” and are advocated for routine use, ignoring the risk/benefit profile that comes with every drug. We performed a comprehensive review of the literature on steroid administration in acute SCI and neurotrauma, including the NASCIS studies to determine the best evidence available for the clinical benefits and risks of administering high-dose steroids in acute SCI. The search explored PubMED, EMBASE, and Cochrane Trials Registry databases through August 2009 and a review of the references that follow these captured articles. A broad search using only the terms “spinal cord injury” and “steroids” returned 1069 articles and 166 reviews. From these articles there were only five randomized, controlled studies using steroids in the treatment of acute SCI,14–19 a meta-analysis,20 and seven prospective and retrospective case studies.23–28,33 Using accepted guidelines in grading the levels of evidence and strength of the study recommendations, the five published randomized trials were evaluated and assigned a level and grade of recommendation. Briefly, the criteria used to evaluate the studies were how well the study was designed, including a clear randomization process, power calculation to determine the minimum number of patients required to detect a statistical difference, an accepted measure of neurological function, and finally follow-up rate. These are summarized in Tables 10.1 and 10.2. Only the randomized, controlled studies will be discussed in depth. Table 10.2 Summary of Retrospective Studies of Use of Steroids in Acute Spinal Cord Injury
Use of Steroids for Spinal Cord Injury
Study | Year | Level III/IV Evidence |
|---|---|---|
Prendergast et al33 | 1994 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
Gerhart et al23 | 1995 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
George et al24 | 1995 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
Poynton et al28 | 1995 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
Levy et al25 | 2000 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
Gerndt et al26 | 1997 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
Heary et al27 | 1997 | III: Negative, no difference in neurological outcome in acute SCI patients receiving high-dose steroids and placebo |
 Does Administration of Steroids in Patients with Acute Spinal Cord Injury Improve Neurological and Functional Outcome?
Does Administration of Steroids in Patients with Acute Spinal Cord Injury Improve Neurological and Functional Outcome?
Level I Data
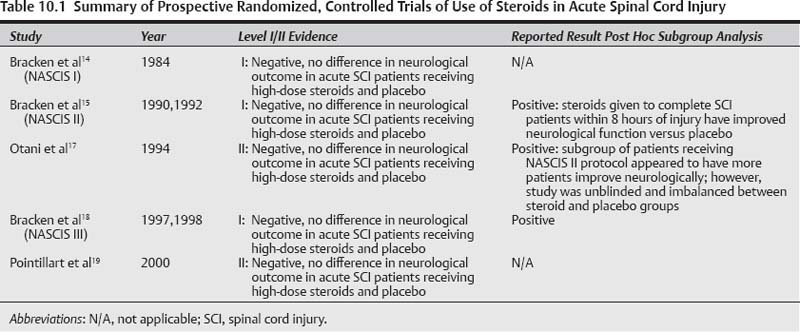
NASCIS I: JAMA 1984;251(1):45–52
The first randomized, controlled trial was published by NASCIS. NASCIS I was a multicenter trial that randomized 330 patients from 1979 to 1981 and compared the clinical effect of methylprednisolone sodium succinate (MPSS) at a “low-dose” regimen of 100 mg/day for 10 days versus a high-dose regimen of 1000 mg/day for 10 days in patients with acute SCI. Neurological motor and sensory recovery was measured in both treatment groups at 6 weeks and 6 months postinjury. A placebo group was not included because it was deemed unethical at the time to deny SCI patients steroids because of the widespread belief in their presumed benefit. Secondary end points to the study included monitoring for adverse effects of steroid administration. The study was well designed with hypothesis-driven research questions and a power calculation requiring a minimum of n = 50 in each arm to measure a significant difference in neurological scores by 7 points. Follow-up was reasonable with 78% at 6 weeks (n = 258, 125 high dose, 133 low dose), and 54% at 6 months (n = 179, 91 high dose, 88 low dose). The numbers of patients evaluated met the minimum number needed as modeled by the power calculation; however, the overall study was weakened due to the significant loss of patients to follow-up. Despite this, NASCIS I is classified as a level I study by evidence-based medicine convention.29–31
NASCIS II: NEJM 1990;322:1405–11
Based on animal models examining the molecular patho-physiology of SCI, there was some evidence that even higher doses of MPSS than those used in the NASCIS I trial might have a significant effect on ameliorating secondary SCI injury through inhibition of the oxygen–lipid peroxidation free radical cascade. NASCIS II was designed to evaluate MPSS administration at these high doses. Given there was no statistical difference between the two low-dose MPSS groups in NASCIS I, the second NASCIS trial justified the inclusion of a randomized placebo control group in addition to the high-dose MPSS. A third agent, high-dose naloxone, which had also been shown to have some benefit in the inhibition of lipid peroxidase pathways in animal models, was also included. Each of the treatments was given within 24 hours of injury, and neurological American Spinal Injury Association (ASIA) grade and neurological motor and sensory scores were followed at 6 weeks and 6 months.
NASCIS II was a multicenter, prospective, randomized, double-blinded trial that enrolled 487 patients with acute SCI into three treatment arms: high-dose MPSS (n = 162), naloxone (n = 154), and placebo (n = 171). The study question was to “determine the safety and efficacy of methylprednisolone and naloxone.” All the patients were captured within 12 hours of their injury; 80% received the drug within protocol time limits, and 92% received the drug dosing according to protocol. There was no disclosure in the original article as to the stratification of when patients received the study drug in each arm of the study; however, in their analysis they chose to divide their patients in each arm into those who received treatment in less than 8 hours or greater than 8 hours. The authors justified the 8-hour time point as part of their original study hypothesis to look at the effects of early steroid treatment and that the 8-hour point coincided with the median time point in which their cohort of study patients received steroids. This arbitrary time point was used similarly to large studies such as the IV-TPA study, in which the 3-hour time point window was arbitrarily chosen and established as the limit to IV-TPA in postocclusive stroke. A post hoc analysis of the data found that the majority of patients received their treatment after the 8-hour window had passed and were thus excluded from further analysis. There was also no power calculation discussed as to the minimum number of patients required to detect a meaningful difference in neurological scores as an entire group or as subgroups divided according to the 8-hour time point.
In addition to the time point division, the authors chose to further divide the patients in each treatment arm by extent of injury—complete versus incomplete (motor and sensory). Finally, the study authors chose to use an unconventional method of analyzing neurological scores by only including motor and sensory scores from one side; there was no intent to evaluate functional recovery. The high follow-up rate for each subgroup at 6 weeks and 6 months for each arm must be acknowledged: MPSS (95%), naloxone (91%), and placebo (95%).
The overall results of the NASCIS II study were negative when all patients randomized to each arm were considered in preplanned analyses and when treated within the 12-hour time period. There were no statistical differences seen in ASIA outcomes or motor or sensory scores between the placebo, MPSS, or naloxone groups. A trend was seen in adverse effects with high-dose MPSS administration, including a 3× increase in pulmonary embolus, 1.5× increase in gastrointestinal (GI) bleeds, and a 2× increase in wound infections.
However, despite the overall negative results, the conclusions and recommendations of the original article were based on a subgroup analysis of patients randomized to each arm that were captured and given the study drug within 8 hours of their injury who had complete SCI. Forty-five patients given MPSS within 8 hours of injury who had complete SCI had on average a 5-point improvement in their unilateral motor and sensory scores when compared with a group of 44 patients given placebo at 6 months postinjury. The authors further contended that when the secondary end points of adverse effects were analyzed for this subgroup, there was no significant difference in mortality or morbidity.
This was heralded as a landmark study that was the first to show through a randomized, controlled study some evidence suggestive of the clinical benefit of high-dose steroids in acute SCI; and given the dearth of medical options, was recommended as an absolute must in all acute SCI patients and published and disseminated as a practice-changing article.
NASCIS III: JAMA 1997;277:1597–604
The momentum garnered from the NASCIS II trial carried over to NASCIS III where the third and final randomized, controlled trial by the NASCIS work group was developed to determine whether there was any benefit in continuing high-dose MPSS infusion for 48 hours after acute SCI. The study compared high-dose MPSS given over 24 hours versus 48 hours to acute SCI patients captured within 8 hours of injury. Given the purported benefit seen in the subgroup analysis of high-dose MPSS, no placebo was included with this trial as it was again felt unethical to deny patients a treatment with an “obvious” clinical benefit.
NASCIS III was also a multicenter, prospective, randomized, double-blind trial. A total of 499 patients who met study criteria were randomized into one of three arms: 24-hour MPSS (n = 166), 48-hour tirilazad mesylate (n = 167), and 48-hour MPSS (n = 166). Unilateral motor and sensory scores were measured as well as functional neurological outcome using the Functional Independence Measure (FIM) at 6 months postinjury. No power calculation was described, but 6-month follow-up was a respectable 87% for the 24-hour MPSS group, 89% for the tirilazad group, and 89% for the 48-hour MPSS group.
When considering the entire cohort of patients randomized to each arm, there was no statistically significant difference in neurological scores between any of the three groups. However, a post hoc analysis in which patients were stratified into those treated within 3 hours versus 3 to 8 hours of injury showed that the 48-hour MPSS patients treated within 3 to 8 hours recovered on average 3.4 motor points compared with 24-hour MPSS patients. There was no difference in sensory improvement. The authors also found a trend toward improvement in functional neurological recovery in the 48-hour MPSS group captured within 3 to 8 hours of injury as shown by an increase of FIM scores, particularly in self-care and sphincter control. It should be noted that even these statistical points of interest were lost after 12 months of follow-up.16
The secondary end points of adverse effects of MPSS for 24 hours versus 48 hours found a significant increase in severe sepsis and pneumonia in the MPSS arm treated for 48 hours. Unlike in NASCIS II where only a trend toward adverse effects was seen, a significant sixfold increase in deaths due to respiratory complications in the 48-hour MPSS group (p = 0.056) was observed. The authors suggest that the neurological benefit gained in acute SCI patients 3 to 8 hours postin-jury who are maintained on MPSS for 48 hours is worth the risk of sepsis and pneumonia; both of which are conditions that may be aggressively treated.
Level II Data
Otani et al: Sekitsui Sekizui: 1994;7:633–647
Following the publication of the NASCIS II trial, Otani et al17 sought to replicate the study findings of administering high-dose MPSS within 8 hours of SCI using the NASCIS II protocol. The Otani study was a multicenter, prospective, randomized, unblinded trial where 158 patients were enrolled, and 116 were available for follow-up (73%). Patients were randomized into two arms where one group was treated with MPSS bolus followed by a 23-hour infusion as per NASCIS II, and the second group was treated with routine medical management of which some patients received steroid therapy, who were subsequently excluded (N = 29) from further analysis. Primary outcomes were neurological improvement using the same neurological assessment used in the NASCIS II study; secondary end points were complications and adverse events. No significant difference in the outcomes between the two groups were seen; however, a post hoc subgroup analysis found a statistically significant increase in the number of patients who had improved sensory scores in the group of patients that received steroids (68%) versus those that received nonsteroidal medical management (32%), although magnitude of recovery was not objectified.
There was no significant difference in secondary adverse events; however, a trend was detected in septic complications in the MPSS group (66%) versus the non-MPSS group (45%). A total of 41 (26%) patients were excluded from the final analyses after randomization primarily within the control group for protocol violations generating an imbalance between the two arms, with the MPSS group having 70 patients versus the nonsteroid group with 40 patients.
Pointillart et al: Spinal Cord 2000:38:71–76
The Pointillart study19 was a single-center, prospective, randomized, double-blind trial that enrolled 106 acute SCI patients that were hospitalized within 8 hours of injury. Patients were randomized into four arms: MPSS bolus 30 mg/kg then 5.4 mg/kg/h for 23 hours, nimodipine bolus 0.5 mg/kg/h for 2 hours then 0.03 mg/kg/h for 7 days, MPSS and nimodipine together, and placebo. The primary purpose of the study was to examine the potential therapeutic benefit of nimodipine compared with MPSS and placebo in acute SCI. One hundred patients were available for follow-up, ~25 patients per arm. No power calculations were provided to guard against type II error. Primary end points were ASIA neurological outcomes, whereas secondary end points were adverse events. The study failed to show any significant difference in ASIA scores between the four arms at 1 year postinjury. No significant difference was seen overall in patients who received steroids in the study (n = 54) and those who did not (n = 52). However, a nonstatistically significant trend toward an increase in septic complications and wound infections was seen in the MPSS group (66% with MPSS, vs 45% without MPSS).
Bracken MB: Meta-Analysis Cochrane Review 2002
A Cochrane Review on the use of steroids in acute SCI identified all steroid and SCI randomized, controlled trials, pooling data to perform a meta-analysis.20 In the latest update of the Cochrane Review the five key studies identified remain unchanged.21 There have only been two other studies attempted; both of which were excluded on the basis of key methodological errors. Of the five studies included, meta-analysis has suggested the only significant difference to arise from 24-hour MPSS administration by pooling data from the NASCIS II, Otani et al,17 and Petitjean22 studies. Overall a motor improvement score of 4 points was found, with the majority of effect stemming from the NASCIS II data.20 From published guidelines on the grading of evidence-based literature, this meta-analysis is weakened by the inhomogeneous nature of results and quality of the studies.29,30 The potential conflict-of-interest generated by a principle investigator performing a meta-analysis of his own research should be acknowledged.
Level III Data
Retrospective, Case Series Studies
There are several retrospective and case series studies that have been published on the use of steroids in acute SCI. These are listed in Table 10.2. The consistent outcome in all of these studies is that they are negative for any favorable effect of administration of high-dose MPSS in acute SCI. Although the quality of the evidence is categorized as level III, the findings are consistent with the overall negative results seen in all five of the published randomized trials.
 Summary
Summary
Using accepted published conventions in grading the level of evidence in the literature28–30; the overwhelming evidence shows that there is no clinical benefit to administering steroids, particularly MPSS in the setting of acute SCI. Of the five randomized clinical trials that were designed to establish a clinical utility for MPSS in SCI, the NASCIS I–III studies were the most well designed and executed and may be considered true level I studies with good enrollment, stringent randomization, blinding, protocol adherence, and 80 to 90% follow-up at all of their follow-up time points. The overall results from these studies showed no clinical benefit of high-dose steroids in acute SCI and instead consistently demonstrated a trend toward adverse effects, including pneumonia, wound infection, sepsis, and death.
Despite the lack of evidence for steroid use in SCI, the authors of the NASCIS II and III studies chose to focus their conclusions and recommendations on post hoc subgroup analyses of arbitrary 3- to 8-hour time points from injury. There is no convention for grading level of evidence in post hoc subgroup analyses within large, well-designed, randomized, controlled trials. It is intuitive that results from such analyses might be noted as interesting but inherently retrospective in nature, and used to develop new hypotheses as the basis for subsequent clinical studies rather than forming the basis of any firm treatment recommendations. Both the NASCIS authors and the medical community at large can be faulted for propagating conclusions and clinical recommendations regarding MPSS administration for SCI on such weak evidence.
Over the past 2 decades the results of the NASCIS trials have been extensively reviewed.31–34 Virtually all proponents for and against the use of high-dose steroids in the setting of acute SCI agree that the overall results of all studies to date are negative. Even at face value the post hoc analyses demonstrate marginal clinical benefits at best with absolute improvements in motor (not sensory) scores of 4 to 5 points and no significant change in meaningful functional outcome at 6 months or at 1 year postinjury. There is also agreement within all studies reviewed of an overall trend toward increased adverse effects among patients receiving high-dose steroids. The possible therapeutic effect of high-dose MPSS in SCI seen in subgroup analyses in the NASCIS II and III trials may be interesting but warrants further investigation using the new standards for study design and analysis that are being established in SCI clinical research. Based on published guidelines for grading studies,29–31 the evidence for clinical improvement using high-dose steroids in acute SCI is weak and therefore cannot be considered a clinical standard nor guideline; however, steroids may be considered as a treatment option by some clinicians keeping in mind the potential adverse effects of MPSS.
Pearls
• Level I, II, and III evidence shows no significant benefit for the use of steroids in acute spinal cord injury.
• Post hoc analyses generated from NASCIS II and III suggest interesting subgroup effects of uncertain significance.
• There is consistent observation of increased risk of severe adverse events related to high-dose steroid administration in acute spinal cord injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree