Modifiable risk factors
Hypertension
Diabetes
Lipids
Cardiac disease (including atrial fibrillation, recent large myocardial infarction, mitral stenosis)
Infective endocarditis
Cigarette smoking
Obesity
Metabolic syndrome
Alcohol consumption
Physical inactivity
Sickle cell disease
Hyperhomocysteinemia
Use of oral contraceptives
Use of illicit drugs
Migraine
Unmodifiable risk factors
Age
Gender
Genetic risk factors
Race/ethnicity
Geographic location
1.1.4 Subtypes and Pathogenesis
The most commonly used classifications of ischemic stroke are based on the presumed mechanism of the focal brain injury, and the type and localization of the vascular lesion. The most commonly used etiopathological classification is TOAST criteria, which categorizes stroke in: (a) large-artery atherosclerotic infarction; (b) embolism from a cardiac source; (c) small-vessel disease; (d) other determined cause (such as dissection, hypercoagulable states, or sickle cell disease); and (e) infarcts of undetermined cause. However, the difficulties in classifying stroke with multiple etiologies led to the development of new classification systems, such as web/evidence-based decision-making. However, among classification systems, the Oxfordshire Community Stroke Project (OCSP), although less used, categorizes stroke syndromes into total anterior circulation infarcts (TACI), partial anterior circulation infarcts (PACI), lacunar infarcts (LACI), and posterior circulation infarcts (POCI), and has been estimated to better predict the prognosis (higher mortality in TACI group, high recurrence in PACI group).
1.1.5 Diagnostic Markers
The diagnosis of stroke/TIA requires a compatible history (an abrupt onset of a clearly focal neurological deficit) and neurological examination (a neurological deficit that can be localized to a specific vascular territory) backed up by neuroimaging excluding mimics.
1.1.5.1 Blood
May reveal predisposing causes, such as polycythemia, sickle cell disease, renal impairment, electrolyte disturbances, and hyper-/hypoglycemia as well as other risk conditions such as hyperlipidemia, hyperhomocysteinemia, or concomitant infections that should be treated since they impair brief-term and long-term prognosis.
1.1.5.2 CSF
Cerebrospinal fluid examination is indicated in some patients for two main reasons:
1.
To exclude other diagnosis such as inflammatory and dysimmune conditions, infections or malignancies
2.
To evaluate new prognostic markers (i.e., orexin A, bradykinin levels)
1.1.5.3 Genetics
Evidence from epidemiological studies in twins, families, and animal models supports the role of genetic factors in stroke pathogenesis and suggests that these factors play a major role in younger age groups and in certain stroke subtypes [6].
Monogenic disorders, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); Fabry disease; cerebral autosomal recessive A arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL); hereditary cerebral amyloid angiopathy (H-CAA); hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS); mitochondrial encephalopathy with lactic acidosis and stroke like episodes (MELAS); and, more recently, COL4A1 syndromes are well-recognized causes of stroke and small-vessel disease. In addition, some inheritable connective disorders have been associated with stroke, particularly with cardioembolic subtype and with stroke caused by epiaortic vessel dissections. Disease progression and long-term prognosis as well as causes of death in these patients are largely unknown. Only one study reported outcome data for CADASIL. The median ages of onset for inability to walk without assistance, bedridness, and death were significantly lower in men than in women (58.9 vs. 62.1; 62.1 vs. 66.5; and 64 vs. 70.7 years, respectively) (all P ≤ 0.01) [7].
However, monogenic diseases explain less than 5 % of all stroke cases. In most cases, stroke is a polygenic disorder for which classic patterns of inheritance cannot be identified [8].
1.1.6 Imaging and Other Investigations
Cerebral CT scan is frequently normal after ischemic stroke.
Cerebral MRI is the investigation of choice in acute ischemic stroke. DWI sequences demonstrate acute lesions in the first few hours after stroke and are much more sensitive than CT scan (Fig. 1.1).
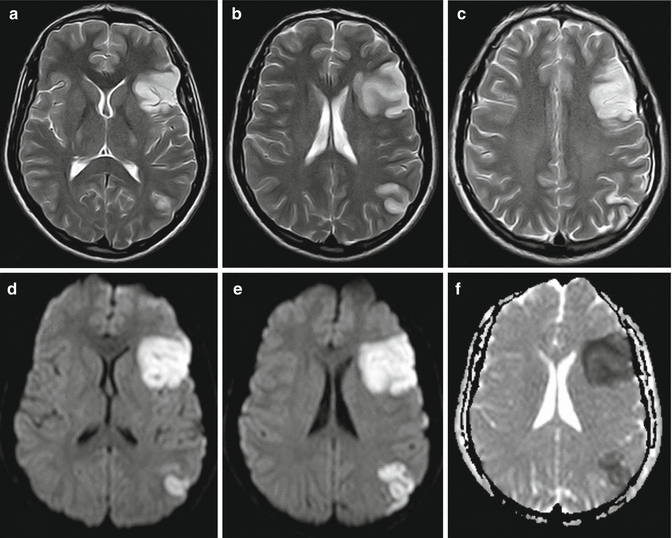
Fig. 1.1
Acute ischemic stroke. Axial T2-weighted images, (a–c), demonstrate cortical-subcortical hyperintesities in the left frontal-opercolar and in the left temporal-parietal carrefour regions, “enhanced” in DWI images, (d, e), and in ADC map, (f)
Arterial imaging with carotid Doppler ultrasonography, computed tomography angiography (CTA), or magnetic resonance angiography (MRA) provide information about the status of intracranial and epiaortic vessels
Electrocardiography is used to detect paroxysmal atrial fibrillation or cardiac ischemic acute disease.
Transthoracic or transesophageal echocardiography is used to detect cardiac sources of embolism other than atrial fibrillation.
1.1.7 Top Differential Diagnoses
Epileptic seizures, toxic/metabolic disorders, syncope/pre-syncope, peripheral vestibulopathy, sepsis including meningoecephalitis, space-occupying lesions/hematoma, multiple sclerosis, migraine/familial hemiplegic migraine.
1.1.8 Principles of Treatment
1.1.8.1 Acute Phase Treatment
Stroke Units (SUs)
The most substantial advance in stroke therapy has been the routine management of patients in stroke units (SUs), with stroke-dedicated beds and staff. SU treatment is effective in reducing mortality and disability in about 20 % of patients, independently from age, stroke subtype, and severity. Although the precise components of SU management responsible for the effectiveness of SUs are unclear, monitoring, early mobilization, and general adherence to best practice could improve stroke outcome [9].
Thrombolysis (Recombinant tPA)
Recombinant tPA (rtPA) is the only evidence-based effective therapy for acute ischemic stroke. The major adverse effect of thrombolysis is symptomatic intracerebral hemorrhage, seen in about 6–7 % of cases, but which is even lower in the European (SITS MOST) study population. Although the publication data of ECASS III study widened the therapeutic time window from 3 to 4.5 h, the number of patients who could receive rtPA treatment is small. However, substantial uncertainties about the efficacy in reducing death and improving functional outcome still remain regarding the longest time window, the efficacy in people older than 80 years, and the role of other potential influencing factors (i.e., stroke subtype, use of antiplatelet before stroke). A recent meta-analysis on 7012 patients seems to support the treatment efficacy independently of age or stroke severity and in at least a part of patients treated within 6 h [10].
Endovascular Treatment (ET)
The available evidence from trials performed with different methodological approaches, including “bridging” and different devices, does not show that endovascular therapy achieves superior outcomes in comparison to intravenous thrombolysis for the acute treatment of ischemic stroke patients [11].
Decompressive Surgery for Ischemic Stroke
Decompressive surgery, removal of part of the skull and duraplasty, has been proposed as a way to control raised intracranial pressure and reduce shifts of swollen brain tissue after stroke. The results of randomized trials show that decompression reduces death rate (ARR 38 %, 15–60; p = 0.002) and slightly improves the functional outcome, defined as an mRS score of 5 or 6 (mRS: 4 moderately severe disability; mRS 5: severe disability) in patients with space-occupying hemispheric infarction treated within 48 h of stroke onset. Seventy-eight percent of survival was observed in patients submitted to decompressive surgery versus 29 % of the medically treated. The modified Rankin Score (mRS) was ≤4 in 75 % of patients in the surgery group versus 24 % in controls; mRS was ≤3 in 43 % versus 21 % in the surgically and conservatively treated group, respectively [12]. There is no evidence that this operation improves functional outcome when it is delayed for up to 96 h after stroke onset. Recent guidelines support the use of decompressive craniotomy in patients with swelling stroke who continue to deteriorate neurologically. Patients older than 60 years also benefit from this treatment [13]. The 3-year follow-up data of HAMLET study, which is the largest randomized trial in this field [14, 15], reported that surgical patients had a lower case fatality rate than controls, whereas the risk of a poor outcome did not differ between groups.
Hypothermia
Pharmacological and physical cooling studies do not reduce the risk of death or dependency (OR 0.9; 95 % CI, 0.6–1.4) in patients with acute stroke [16].
1.1.9 Secondary Prevention of Stroke
1.1.9.1 Oral Antiplatelet Drugs
The overall use of antiplatelet agents in secondary prevention trials has been shown to provide about 22 % of relative risk reduction of stroke recurrence.
A recent meta-analysis, including 8 trials and 41,483 patients, showed that aspirin therapy at 160–300 mg daily, started within 48 h after ischemic stroke onset, reduces the risk of early recurrent ischemic stroke (OR 0.77; 95 % CI 0.69, 0.87) without a major risk of early hemorrhagic complications. However, clinical and laboratory evidence demonstrates diminished or no response to ASA in some patients, who labeled ASA resistance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




