and Aditya G. Shivane1
(1)
Cellular and Anatomical Pathology Level 4, Derriford Hospital, Plymouth, UK
Abstract
Diseases affecting the blood vessels of the CNS constitute an important group and are associated with significant morbidity and mortality. This chapter starts with a basic discussion of CNS vascular anatomy before going into specific diseases. Atherosclerosis is the most common vascular disease and is a major cause for stroke and vascular dementia. The majority of vascular diseases are sporadic and multifactorial, but a few rare hereditary forms also occur such as CADASIL and familial amyloid angiopathies. Vasculitides form an important group of treatable conditions which also pose considerable diagnostic challenge for both clinician and pathologist. The end result of most vascular disease is ischaemia, infarction or haemorrhage.
Keywords
Blood vesselMalformationInflammationInfarctionHaemorrhageThe brain is a highly metabolically active organ and depends largely on aerobic metabolism for its survival with very little capacity for anaerobic metabolism. This high demand is reflected in the cerebral blood flow which is 20 % of the cardiac output, even though the brain makes up only 2 % of the body weight. The blood vessels ensure delivery of a continuous supply of important nutrients like oxygen and glucose to tissues of the nervous system. Even a short interruption of blood flow to the brain results in disastrous consequences. The diseases which affect the nervous system vasculature can cause either (1) global or focal interruption to blood flow leading to ischaemia and infarction, or (2) leakage or rupture of blood vessels leading to haemorrhage.
4.1 Vascular Anatomy
4.1.1 Arterial Supply
Two major pairs of arteries supply blood to the brain, the internal carotid arteries (anterior circulation) and vertebral arteries (posterior circulation). The anterior circulation carries roughly 70–80 % of blood supply and the posterior circulation the remaining 20–30 %. The internal carotid arteries (ICA) branch off from the common carotid arteries in the neck and enter the cranial cavity. They pass through the cavernous sinus and then divide into two branches, the middle cerebral arteries (MCA) and anterior cerebral arteries (ACA). The vertebral arteries (VA) arise from the subclavian and brachiocephalic arteries and pass through the transverse foramina of C1-C6 vertebrae and enter the cranial cavity via the foramen magnum. The largest branch of VA is posterior inferior cerebellar artery (PicA). Along the ventral surface of the brainstem the two VA join to form the basilar artery which then divides into two posterior cerebral arteries (PCA). The two ACA are joined by anterior communicating artery (AComA). The PCA join with the ICA via posterior communicating arteries (PComA) to complete the circle of Willis (Fig. 4.1). There is considerable anatomical variation in the arteries forming the circle of Willis. Anastomoses within the circle of Willis allow collateral supply of blood in the event of arterial blockage.
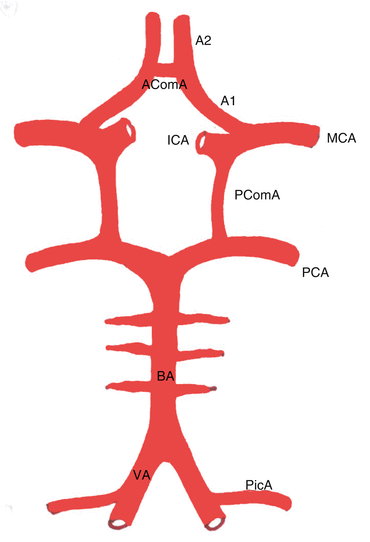

Fig. 4.1
Diagram of Circle of Willis showing all the major branches. A1, A2 segments of anterior cerebral artery, AComA anterior communicating artery, MCA middle cerebral artery, ICA internal carotid artery, PCA posterior cerebral artery, PComA posterior communicating artery, BA basilar artery, VA vertebral artery, and PicA posterior inferior cerebellar artery
The branches of the MCA supplies most of the lateral aspect of the brain and deeper structures like striatum, internal capsule, hippocampus, amygdala, optic radiation and choroid plexus. Occlusion of MCA or one of its key perforating branches produces the ‘classic hemiplegic stroke’ affecting the putamen and internal capsule. The ACA supplies the medial aspect of frontal and parietal lobes, anterior limb of internal capsule and corpus callosum. The branches of PCA supply the inferior and medial aspects of temporal and occipital lobes, thalamus and posterior limb of internal capsule. Branches from the VA, basilar artery and PCA supply the entire brainstem and cerebellum.
The blood supply to the spinal cord is by the branches of VA- the two posterior spinal arteries (PSA) (posterior third of the cord) and a single anterior spinal artery (ASA) (anterior two-thirds of the cord) (Fig. 4.2a, b). The cord is also reinforced by radicular arteries arising from segmental vessels including the ascending cervical, intercostal and lumbar arteries.
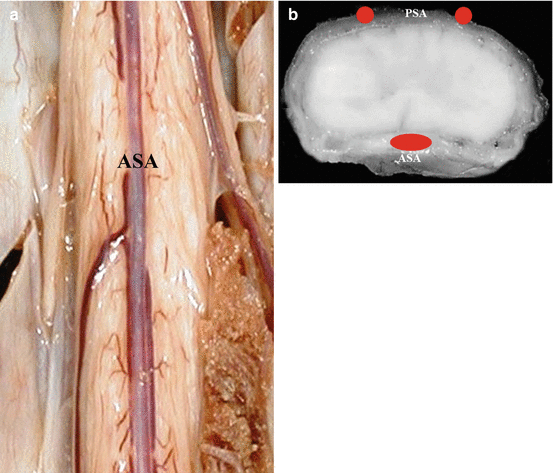

Fig. 4.2
(a, b) Vascular supply of spinal cord. ASA anterior spinal artery, PSA posterior spinal artery
4.1.2 Venous Drainage
The venous drainage is through superficial and deep veins. The superficial veins in the subarachnoid space empty into the venous sinuses. The upper part of the brain drains into superior sagittal sinus, the middle part into the cavernous sinus and the lower part into the transverse sinus. The deep veins drain into the great cerebral vein of Galen and through straight sinus empties into the transverse sinus. The blood from transverse sinuses drains to sigmoid sinus, internal jugular vein and then to the heart through brachiocephalic vein and superior vena cava.
The anterior and posterior spinal veins drain blood from the cord into the vertebral venous plexus. The blood from plexus drains into lumbar veins, azygos and hemiazygos veins.
4.2 Diseases Affecting the Blood Vessels
4.2.1 Atherosclerosis
Atherosclerosis is the most common vascular disease and mainly affects large and medium-sized muscular and elastic arteries. It is also the most important cause for ischaemic stroke. In the intracranial compartment, the internal carotid and basilar arteries are the most severely affected. The risk factors for atherosclerosis include- increasing age, male gender, hyperlipidaemia, gene polymorphisms (ABCA1, MMP–3, IL–6 genes) [1–3], hypertension, cigarette smoking and diabetes.
Over the years several theories have been postulated for the formation of the atherosclerotic plaque or atheroma (‘Atheroma’ = Greek word for ‘Gruel’). These include- (1) cellular proliferation within the intima, (2) organisation and growth of thrombi, and (3) vascular injury or response to injury theory. The latter is currently the favoured hypothesis. The endothelium can become dysfunctional or injured by various factors such as mechanical, excess lipid, toxins, bacterial and viral infections, haemodynamic stress and immune reactions. The injured endothelium attracts monocytes circulating in the blood and also the smooth muscle cells from the media into the intima. Lipids also accumulate within the vessel wall during this process. The damaged endothelium release growth factors which help in the proliferation of smooth muscle cells eventually culminating in plaque formation (Table 4.1). The plaque can undergo disruption and precipitate thrombus formation that can further obstruct blood flow.
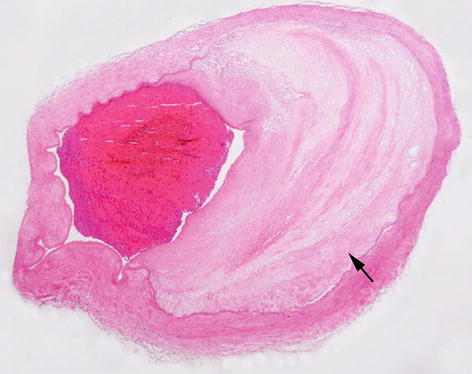
Table 4.1
Morphology of plaques
Lesion | Description |
|---|---|
Fatty streak | Earliest lesion (even seen in children as young as 10 years). Appear as yellow flat lesions. Contain lipid-filled foam cells. Do not cause any disturbance to blood flow. |
Fibrofatty plaque (Fig. 4.3) | Appear as white to pale yellow raised lesion containing lipid in the core and covered by firm white fibrous cap. These lesions impinge on the vascular lumen, are usually eccentric and patchy along the length of the vessel. |
Complicated plaque | A fibrofatty plaque which shows evidence of rupture, ulceration or erosion, haemorrhage, thrombus formation or aneurysmal dilatation. |

Fig. 4.3
A large artery showing intimal fibro-fatty atheromatous plaque (arrow) and luminal narrowing. H&E stain
4.2.2 Small Vessel Disease
As the name implies, small vessel disease (SVD) affects mainly the small perforating arteries or arterioles, venules and capillaries. The compromise of blood supply due to SVD can result in distinct disorders which include- lacunar infarcts (small infarcts ≤1.0 cm), vascular dementia and primary non-traumatic parenchymal brain haemorrhage. The risk factors associated with SVD include age, diabetes and hypertension. The MRI scan of brain typically shows symmetric, multifocal abnormality within the white matter and basal ganglia. Small blood vessels in the brain can be affected in vast number of diseases such as inflammatory, infective, toxic and hereditary disorders, but the three common conditions include arteriolosclerosis (including Binswanger’s disease), cerebral amyloid angiopathy (CAA) and cerebral autosomal dominant arteriopathy with sub-cortical infarcts and leukoencephalopathy (CADASIL). Binswanger’s disease is a sporadic condition affecting elderly hypertensive patients. The pathology is characterised by arteriolosclerosis and multiple white matter infarcts. Table 4.2 describes the pathological features of SVD.
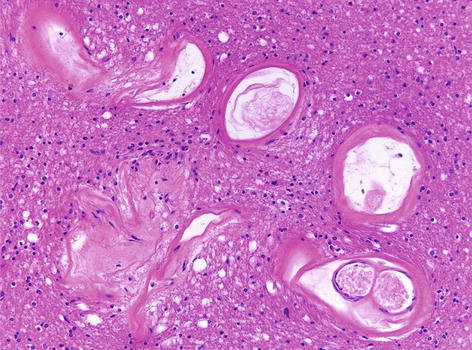
Table 4.2
Pathological features of small vessel disease
Lesion | Description |
|---|---|
Atherosclerosis | Atheromas similar to those seen in the larger blood vessels. Because of the small size of the vessels affected, even a small atheroma can compromise the lumen considerably. |
Fibrinoid necrosis and Lipohyalinosis | Fibrinoid necrosis refers to the early stage where the leakage of blood–brain barrier results in deposits of plasma proteins including fibrin in the vessel wall. The vessels at this stage are vulnerable to rupture. At a later stage, the wall will be replaced by acellular fibrosis with little or no lipid termed ‘lipohyalinosis’. |
Arteriolosclerosis (Fig. 4.4) | Hyaline thickening of the arterioles. Commonly associated with clinical vascular dementia. |
Micro-aneurysms | Now believed to be ‘complex tortuosities’ of blood vessels and the causation is unclear [4]. |

Fig. 4.4
Small blood vessels within the white matter showing prominent thickening and hyalinisation suggestive of small vessel disease. H&E stain
4.2.3 Amyloid Angiopathy
The cerebral amyloid angiopathy (CAA) is characterised by the deposition of fibrillar amyloid protein (Box 4.1) in the walls of blood vessels of the brain and meninges. There are several different amyloidogenic proteins (Table 4.3).
Table 4.3
The amyloid angiopathies
Disease | Gene involved/precursor protein | Type of amyloid |
|---|---|---|
Sporadic Aβ-CAA (commonly associated with sporadic Alzheimer’s disease) | APP/Amyloid precursor protein | Aβ |
Hereditary Aβ CAA (Dutch and Flemish types, Familial Alzheimer’s disease) | APP/Amyloid precursor protein | Aβ |
Hereditary cerebral haemorrhage with amyloid angiopathy- Icelandic type | CYST C/Cystatin C | ACys |
Familial amyloidosis- Finnish type | GEL/Gelsolin | AGel |
Familial British Dementia | BRI2/ABri | ABri |
Familial Danish Dementia | BRI2/ADan | ADan |
Familial Amyloidotic Polyneuropathy/Meningovascular amyloidosis | TTR/Transthyretin | ATTR |
Prion protein CAA | PRNP | APrP |
Box 4.1 Characteristics of Amyloid
Misfolded protein which acquires β-pleated secondary structure
Highly insoluble
Binds stains such as Congo red (apple-green birefringence under polarised light) and Thioflavin-S or T (fluorescence)
Can be demonstrated by immunohistochemistry using an antibody to β-amyloid or other amyloidogenic proteins
The sporadic form of CAA is more common than hereditary CAA subtypes, and is composed of Aβ amyloid. Sporadic Aβ-CAA is the most common cause of lobar haemorrhage in the elderly. The prevalence of CAA is high in patients with Alzheimer’s disease (>90 %) and they also share some of the risk factors (genetic polymorphisms in ApoE, PS1, α-1 anti-chymotrypsin, neprilysin) [5]. Some of the other conditions associated with Aβ deposition include Downs syndrome, Dementia pugilistica and vascular malformations.
The macroscopic examination of brain with sporadic Aβ-CAA may show lobar haemorrhages mainly in the frontal or fronto-parietal lobes. Cerebellar haemorrhages are infrequent. There may be changes of associated Alzheimer’s disease, petechial haemorrhages and small infarcts in the grey or white matter. The small to medium-sized arteries/arterioles in the leptomeninges and cortex are preferentially affected. The amyloid deposition is more severe in the posterior cerebrum (parietal and occipital lobes). The affected blood vessels appear rounded and thickened, show deposition of amyloid within the tunica media and adventitia (Fig. 4.5a). The amyloid can be confirmed to be Aβ using immunohistochemistry (Fig. 4.5b). In some cases, the Aβ-laden blood vessel can show giant cell/granulomatous reaction which is termed ‘Aβ-related angiitis’.
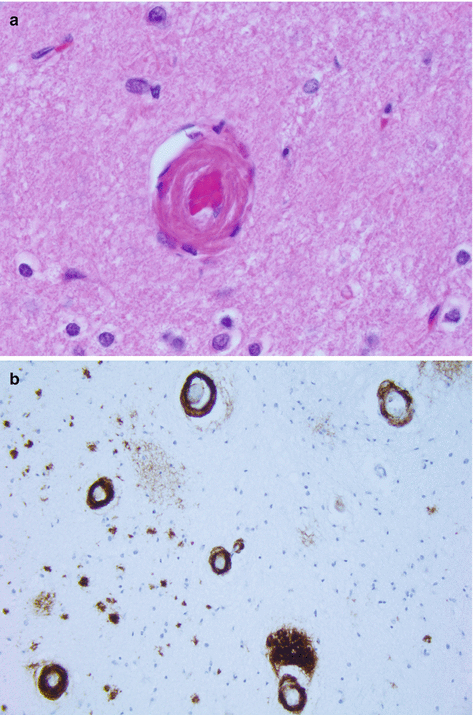

Fig. 4.5
(a) Cerebral amyloid angiopathy showing a thickened cortical blood vessel, H&E stain, (b) cortical blood vessels showing beta-amyloid deposition within their walls, Beta-amyloid immunohistochemistry
4.2.4 Hereditary Vascular Diseases
CADASIL is the acronym for Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, which was first described by van Bogaert in 1955. The defective gene was identified in 1996 to be Notch3 located on chromosome 19. This is a slowly progressive vascular disease equally affecting males and females with onset of stroke before the age of 30 years; the clinical presentation peaks around 40–50 years of age. The classic symptoms include- migraine with aura, ischaemic attacks, psychiatric symptoms, cognitive decline and dementia and rarely seizures. Hyperintensities on T2 weighted and flair MRI within the temporal poles is diagnostic of CADASIL. This disease affects small and medium-sized arteries, and rarely veins, of almost all organs. Therefore, skin biopsy is very useful for the diagnosis of CADASIL. The blood vessels become markedly thickened and fibrotic with accumulation of basophilic, PAS-positive granular material within the tunica media. There is accompanying degeneration of vascular smooth muscle cells. In the brain, the vessels in the leptomeninges and white matter are preferentially affected. The narrowing of vascular lumina results in ischaemic lesions mainly in the white matter, deep grey structures, brainstem and rarely in the spinal cord, but spares the cortical grey matter. The ultrastructural examination of the arteries reveals destruction of smooth muscle cells and accumulation of granular osmiophilic material (GOM), pathognomonic of this disease. An antibody against the extracellular domain of Notch3 is available and may be useful in making a diagnosis [6]. Majority of CADASIL cases demonstrate missense point mutations in the Notch3 gene with more than 130 different mutations identified so far. The pathogenesis of this disease is still unclear, but it is thought that the mutated Notch3 protein accumulation is likely to exert a toxic effect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








