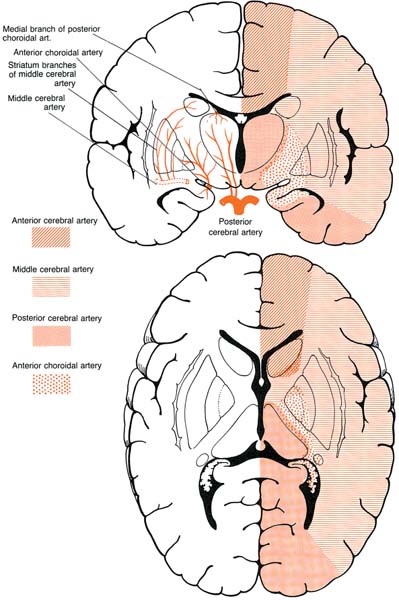
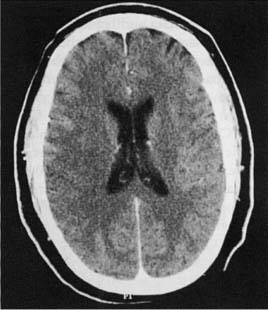
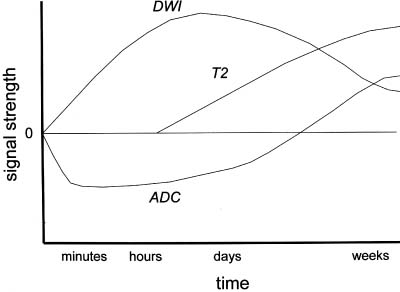
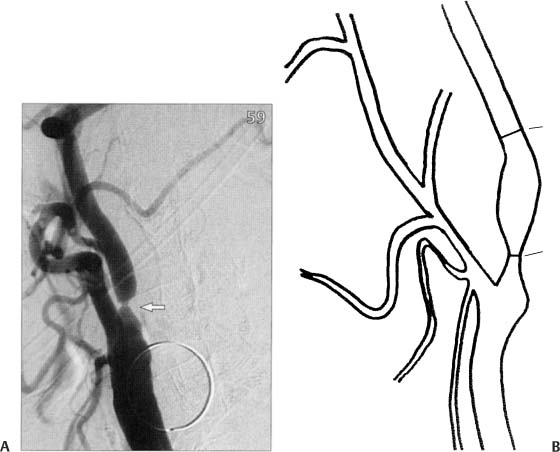
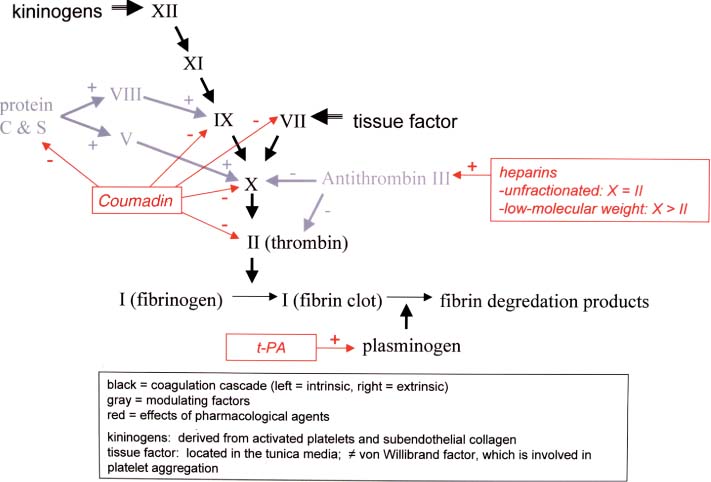
2 Note: Significant diseases are indicated in bold and syndromes in italics. 1. Subtypes: considering all ischemic strokes a. 30% are likely due to small artery occlusion b. 30% are likely due to large artery thromboembolism c. 20% are likely due to cardioembolism d. 20% are due to other mechanisms or are cryptogenic 2. Epidemiology: ischemic stroke accounts for 80% of all strokes a. age: the most predictive factor for stroke b. sex: male predominant disease until age > 75 c. race: Blacks and Hispanics have higher stroke rates 3. Risk factors: identification of a risk factor that may have caused the stroke (e.g., atrial fibrillation) does not obviate the need to evaluate the patient for other possible risk factors a. previous stroke, either clinical or radiographic b. transient ischemic attack (TIA): 10% 90-day stroke risk, and 25% 90-day risk of TIA, stroke, or myocardial infarction i. stroke risk is highest within 24–48 hours of TIA, which supports the recommendation of hospital admission and aggressive evaluation of TIA patients c. intracranial atherosclerosis: 7%/year stroke risk; higher incidence in Blacks, Asians, and Hispanics d. hypertension: systolic pressures > 140 mmHg and diastolic pressures > 90 mm Hg are independent risk factors for stroke, and stroke risk is proportionate to the degree of hypertension e. carotid stenosis: the degree of stenosis is proportionate to stroke risk in both symptomatic and asymptomatic patients f. heart disease (Box 2.1) Cardiovascular Procedure Stroke Risks i. atrial fibrillation (1) overall stroke risk = 6%/year; stroke risk is higher in chronic atrial fibrillation or atrial fibrillation due to thyroid disease (a) 1%/year stroke risk in patients < 65 years old without other risk factors (can be treated with aspirin if need be) (b) 7–18%/year stroke risk in patients with at least one other stroke risk factor (must use Coumadin) (2) stroke risk is highest at the time of onset of atrial fibrillation; conversion to sinus rhythm without anticoagulation is also high risk (3) atrial enlargement without fibrillation is also a stroke risk factor ii. congestive heart failure: stroke risk ≈ 4%/year iii. myocardial infarction: wall motion abnormalities (particularly of the anterior wall) allow intracardiac thrombus formation that is a source of emboli (1) mitral valve prolapse is unlikely to be a cause of stroke (2) stroke risk for mechanical valves is greater than for bioprosthetic valves v. patent foramen ovale: may allow a paradoxical embolism from the systemic venous system (e.g., a deep venous thrombosis) causing stroke (1) patent foramen ovale is not conclusively a risk factor for stroke, although it is a risk factor when it is associated with an atrial septal aneurysm (2) a patent foramen ovale is present in 20% of the general population g. aortic arch calcifications and atheroma, particularly if > 4-mm thick or mobile h. cigarette smoking: stroke risk is proportionate to the amount of smoking; after cessation, the stroke risk reduces to nonsmoker’s level within 3 years i. in addition to promoting atherosclerosis, cigarette smoking increases fibrinogen levels leading to a hypercoagulable state i. dyslipidemia i. low-density lipoprotein (LDL) > 100 mg/dL, although the benefit of treating an LDL between 100–130 mg/dL in patients without other risk factors is unknown ii. high-density lipoprotein (HDL) < 40 mg/dL iii. triglycerides > 200 mg/dL j. elevated homocysteine level: risk is proportionate to level, starting at > 10 μmol/L k. diabetes: a risk factor for all types of stroke, yet adequate glycemic control is known to reduce small vessel complications (e.g., retinopathy, nephropathy) but not large vessel complications l. alcohol use: heavy use (>4 drinks/day) has an inconsistent association with stroke that may be race dependent; low-to-moderate use (1–2 drinks/day) may reduce the risk for stroke, likely by improving the lipid profile m. estrogen use i. contraceptives: high-dose estrogen (> 50 μg) contraceptives increase stroke risk likely because they increase blood coagulability and blood pressure; lower dose estrogen contraceptives may also increase stroke risk (1) increased stroke risk is most marked in women > 35 years old who smoke (2) oral or injectable progesterone-only contraceptives have no apparent effect on stroke risk on their own, but may increase stroke risk in women with hypertension ii. hormone replacement therapy: estrogen plus progesterone replacement therapy increases stroke risk in postmenopausal women n. life-style factors: obesity, physical inactivity, diet, and psychological stress all are likely covariates with other risk factors 4. Symptoms: Focal neurological deficits, by definition > 24 hours in duration for stroke and < 24 hours in duration for TIA, although most TIAs last < 15 minutes; speed of onset is generally considered to be acute, however rarely may develop over hours or days a. headache: occurs in 40% of strokes; often stroke is preceded by a sentinel headache i. headache is likely related to ischemia of the blood vessels or the meninges (Box 2.2) b. seizure occurring concurrently with the stroke (6%) i. risk of developing epilepsy is greater if the first seizure occurs > 2 weeks after the stroke 5. Diagnostic testing a. infarction (Fig. 2–1) i. computerized tomography: acute infarction may be identified by hypo-densities at the interface of the gray and white matter (e.g., the insula and extreme capsule, the basal ganglia, and internal capsule) or by sulcal effacement (Fig. 2–2); however, a CT scan within the first 3 hours is often normal ii. magnetic resonance imaging (MRI): diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping identify ≈ 90% of acute infarctions (Fig. 2–3) (1) DWI: a T2-like sequence that increases signal strength in areas where water molecules are not free to diffuse in the local environment (a) during infarction, the failure of membrane ion transports may prevent transmembrane water movement and cause cellular swelling (e.g., cytotoxic edema) that reduces the extracellular space, thereby limiting the diffusion of water (2) ADC: a calculated value that essentially accounts for any underlying increase in T2 signal (e.g., from vasogenic edema, as caused by tumors or inflammation) by subtracting the T2 signal from the DWI signal (3) an increase in T2 signal and a decrease in T1 signal indicate tissue loss, and are not considered accurate for identifying infarction within 8 hours of onset Figure 2–1 Schematic of the arterial territories of the brain. (From Duus P. Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:309, Fig. 8.39. Reprinted by permission.) iii. hemorrhagic conversion of an ischemic infarction occurs in 20% by 3-weeks poststroke (1) typically develops in a gyral pattern (2) clinical deterioration tends to occur when the hemorrhage is > 30% of the infarcted area or when it exhibits mass effect b. TIA: ~ 40% exhibit neuroanatomically relevant DWI and ADC changes despite symptomatic resolution c. carotid artery stenosis (Fig. 2–4) i. for the purpose of identifying endarterectomy candidates, cerebral angiography should be used, although computed tomography angiography (CTA) provides essentially the same measures (1) when not using angiography, carotid Doppler ultrasound and magnetic resonance angiography (MRA) are usually used together because either alone can overestimate the degree of stenosis in comparison with angiography i. transesophageal echocardiography (TEE) is better than transthoracic echocardiography (TTE) specifically for left atrial appendage, septal abnormalities (e.g., patent foramen ovale), and aortic abnormalities; TEE also has a higher accuracy for overall detection of cardiac abnormalities when compared with TTE ii. paradoxical embolism: evaluation for right-left shunt requires intravenous bubble contrast or Doppler echocardiography or transcranial Doppler ultrasound study e. serologies i. for patients > 45 years old: evaluation of risk factors; when suspecting temporal arteritis in patients > 50 years old include sedimentation rate and C-reactive protein serologies ii. for patients > 45 years old: evaluation of risk factors, as per older patients; consider evaluation for antiphospholipid antibodies, coagulation cascade disorders, and inherited stroke disorders Figure 2–2 Early ischemic infarction demonstrating loss of gray-white differentiation in the right hemisphere, particularly around the basal ganglia. (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neuro-surgery. Stuttgart, Germany: Georg Thieme; 2003:45, Fig. 36b. Reprinted by permission.) 6. Treatment a. acute setting i. thrombolytic treatments (1) tissue plasminogen activator (tPA): if given by IV within 3 hours from the time the patient was last known to be normal; relative risk reduction for disability at 3 months = 30% (a) exclusion criteria (i) absolute 1. occurrence of a seizure at the time of presentation 2. history of any intracranial hemorrhage or of a known vascular malformation, evidence of intracranial hemorrhage on CT, or clinical suspicion of intracranial hemorrhage irrespective of CT findings 3. active internal bleeding or arterial puncture at a noncompressible site within the preceding 7 days 4. gastrointestinal or urinary tract hemorrhage within 3 weeks 5. any neurosurgical procedure, major head trauma, or stroke within 3 months 6. blood pressure consistently > 185/110 mmHg or that requires aggressive management to reduce to that level 7. blood glucose < 50 or > 400 mg/dL 8. abnormal prothrombin time or partial thromboplastin time; platelet count < 100,000 (ii) relative: age < 18; possibility of endocarditis or pericarditis; recent lumbar puncture; rapidly improving condition; obtundation or coma (b) complications: 6% intracranial hemorrhage risk, proportionate to neurological impairment and infarct size but not to early CT findings of infarction Figure 2–3 Time course of the change in diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping after cerebral infarction. Figure 2–4 (A) Measurement of carotid stenosis (arrow in the angiogram image) according to the NASCET criteria. The minimal lumen diameter is divided by the diameter of the distal ICA where the walls become parallel to avoid any poststenosis dilatation (B). (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2003:67, Fig. 59 and Loftus CM and Kresowik TF. Carotid Artery Surgery. Stuttgart, Germany: Georg Thieme; 2000:28, Fig. 2-18. Reprinted by permission.) (2) intraarterial prourokinase: relative risk reduction for disability of 35% at 3 months in patients with MCA occlusion when administered within 6 hours of symptomatic onset; t-PA has been substituted for prourokinase (which is not FDA-approved) and intraarterial thrombolytics have been used for occlusion of other major arteries, but without proof of efficacy (a) 10% risk of intracranial hemorrhage ii. carotid endarterectomy: may be performed on an urgent basis in patients with TIAs that are referable to a severe stenosis iii. anticoagulation and antiplatelet drugs (Fig. 2–5) (1) aspirin: given acutely, relative risk reduction of recurrent stroke within 14 days = 30%; also has small benefit in terms of mortality (a) do not give aspirin (or other antiplatelet or anticoagulant agents) within 24 hours of thrombolytic administration (2) heparin: reduces stroke recurrence but increases hemorrhage rate, therefore does not influence overall outcome when given in the acute setting (a) sometimes given in cases with suspected cardiac sources of embolization until Coumadin can achieve clinical effectiveness iv. blood pressure management: elevated blood pressures following a stroke are associated with better outcomes, thus hypertension is usually left untreated 2–3 days poststroke unless systolic blood pressure (SBP) > 220, diastolic blood pressure (DBP) > 130, or mean arterial pressure (MAP) > 130 mmHg (1) avoid using clonidine or diazoxide because they reduce cerebral blood flow (2) blood pressure typically begins to fall on its own after 3–4 days Figure 2–5 The coagulation cascade and sites of anticoagulant action. v. hyperglycemia management: elevated blood glucose is associated with worsened outcomes in stroke patients irrespective of the patient’s diabetic status vi. seizure prophylaxis: no indication in the absence of a seizure (1) with a single seizure occurring less than 2 weeks from the time of stroke, treat with antiepileptics for 3–6 months before considering discontinuation (low risk of epilepsy) (2) for a single seizure > 2 weeks from the time of stroke or for multiple seizures, treat for 2–3 years before considering discontinuation (high risk of epilepsy) b. subacute to chronic setting, and preventative measures i. deep vein thrombosis (DVT) prophylaxis, as stroke patients are at particularly high risk for DVTs ii. evaluation for cardiac ischemia, although large strokes cause transient ischemic-like EKG changes iii. control of intracranial pressure: edema is maximal 3–5 days after large strokes, although it may be asymptomatic iv. anticoagulation and antiplatelet drugs (1) aspirin: relative risk reduction for recurrent stroke ≈ 25% with 75–325 mg qd (once daily) (a) irreversibly inhibits cyclooxygenase production of thromboxane that causes platelet aggregation, but also inhibits vasodilatory prostacyclin production (b) low-dose (e.g., 81 mg qd) or enteric-coated aspirin will inhibit platelet aggregation in only 40% of patients, in comparison with 70% of patients taking higher doses or uncoated aspirin (2) dipyridamole + aspirin (Aggrenox): modestly better than aspirin alone (relative risk reduction for stroke ≈ 30%); high side-effect rate, particularly headaches
Vascular Diseases of the Nervous System
I. Ischemic Stroke
Box 2.1
 Angiography = 1%
Angiography = 1%
 Coronary artery bypass graft = 2%
Coronary artery bypass graft = 2%
 Thoracic aorta operation = 7% (higher incidence in emergent operations)
Thoracic aorta operation = 7% (higher incidence in emergent operations)
| Coumadin is better than aspirin | Aspirin can be sufficient |
Established | Intracardiac thrombus Artificial heart valve | Atrial fibrillation without other stroke risk factors {lone atrial fibrillation} |
| Atrial fibrillation with other stroke risk factors |
|
Limited evidence | Significant aortic plaque | Patients at risk for bleeding (e.g., fall risk) |
| Large areas of cardiac hypokinesis |
|
(a) dipyridamole reduces platelet aggregation by inhibiting cAMP-phosphodiesterase and by blocking adenosine reuptake
(3) ticlopidine (Ticlid): relative risk reduction for stroke ≈ 25%; used in patients who cannot tolerate aspirin, but has a high side-effect rate (diarrhea, neutropenia [1%], thrombotic thrombocytopenic purpura)
(a) reduces platelet aggregation through a poorly understood mechanism
(4) clopidogrel (Plavix): equivalent efficacy against stroke in comparison with aspirin; benefit of clopidogrel over aspirin is its superior protection against myocardial infarction
(a) reduces platelet aggregation by blocking ADP binding to adenosine receptors
(b) same side-effect profile as ticlopidine; fewer hemorrhagic side-effects in comparison with aspirin
(5) Coumadin: 67% stroke risk reduction of stroke from atrial fibrillation when international normalized ratio (INR) is 2.0–3.0 (Table 2–1)
(a) for mechanical heart valves, target INR 3.0–4.0; for bioprosthetic heart valve, target INR 2.0–3.0
(b) side effects
(i) hemorrhage (usually gastrointestinal) ≈ 7%/year with INR > 3.0; hemorrhage risk increases with age > 80 years old
(ii) skin necrosis, usually occurring at the initiation of Coumadin therapy (rare)
(iii) blue toe syndrome: ischemia of the extremities caused by embolization of cardiac material
(6) heparin: no proven benefit in stroke management; used commonly as a bridge to Coumadin therapy and for temporary management of unstable major artery stenosis prior to surgical or endovascular intervention
(a) side effects: heparin-induced thrombocytopenia (HIT) syndromes
(i) type I HIT: develops < 5 days after starting treatment and is clinically benign; heparin causes platelet aggregation, thereby increasing blood viscosity
(ii) type II HIT: develops 1–2 weeks after starting treatment and is associated with arterial and venous thromboses; heparin-induced autoantibodies cause thrombocytopenia but also a paradoxical hypercoagulable state
v. blood pressure management: reduction to a blood pressure < 140/90 mmHg or as low as is tolerated
(1) dietary modification and salt restriction, exercise, and reduction in alcohol consumption should be attempted in conjunction with medication treatment
(3) treat isolated systolic or diastolic hypertension
vi. dyslipidemia management: as with hypertension treatment, the particular drug may not be as important as the degree of lipid lowering that it achieves
(1) HMG-CoA reductase inhibitors/statin agents: relative-risk reduction for recurrent stroke ≈ 25%
(a) have demonstrated ability to reduce arterial plaque size over time, and may have particular benefit in stroke prophylaxis in cases where aortic plaque is a suspected source of embolization
(2) other agents (clofibrate, cholestyramine, gemfibrozil, niacin) and dietary modification generally do not lower lipid levels as much as statin agents
vii. homocysteine reduction: reduction with vitamin therapy (i.e., vitamin B1 + vitamin B12 + folate) has an unclear effect on recurrent stroke prophylaxis, but reduction is certainly beneficial in inherited disorders of homocysteine metabolism (see p. 72)
viii. control of hyperglycemia: no proven benefit in stroke prevention; considerable evidence exists that small-vessel disease in other organs (e.g., retinopathy, nephropathy) is reduced with strict glycemic control
ix. carotid endarterectomy (Box 2.3)
(1) in some studies, asymptomatic stenosis of 60–99% has a 55% relative risk reduction for stroke if operative mortality < 3% and if the patient has a 5-year life expectancy; untreated stroke risk of asymptomatic stenosis is 1%/year
(2) symptomatic stenosis of 70–99% has a 65% relative risk reduction for stroke if operative mortality < 6% and if the patient has a 5-year life expectancy
(a) untreated stroke risk of symptomatic stenosis is 13%/year
(b) benefit of surgery persists for only less than 1 year after stroke or TIA, therefore surgery is best done as soon as possible after a stroke (usually within 4–6 weeks)
(c) 50–70% symptomatic stenosis may also benefit from surgery in
(i) male patients
(ii) conjunction with plaque ulceration
(iii) cases involving nonlacunar hemispheric stroke or TIA
(d) endovascular stenting appears as effective as endarterectomy, with a greater restenosis rate but fewer procedural complications
II. Subarachnoid Hemorrhage
1. Causes
a. aneurysms: account for 80% of all nontraumatic subarachnoid hemorrhage (SAH)
b. other vascular malformations: arteriovenous malformations (AVM); moyamoya disease
c. head trauma: the most common cause of SAH
d. venous or capillary bleeding into the prepontine; and/or ambient cisterns {perimesencephalic hemorrhage}; not associated with aneurysms
e. venous thrombosis
2. Risk factors: as per aneurysms (see p. 77), but also
a. hypertension
b. coagulopathy/anticoagulant use
c. smoking
d. drug use, particularly cocaine and amphetamines: suspecting drug abuse as the cause of SAH does not eliminate the need to search for a vascular malformation
e. estrogen use: high-dose preparations (e.g., birth control pills) may increased SAH risk; no effect of hormone replacement therapy
3. Epidemiology: accounts for ~ 10% of all strokes
a. exhibits a familial tendency that is likely related to an inherited predisposition to aneurysm formation
4. Symptoms: pronounced headache, nausea, and stupor ± focal neurological deficits; severity of nonfocal symptoms in SAH is generally greater than in intracranial hemorrhage
a. seizure at the time of SAH (5%)
b.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree