Introduction
Acute brain ischaemia is the expression of primary vascular, and not brain, pathology. Accurate diagnosis of the vessel pathology is urgent and essential if appropriate timely treatment is to be given. This chapter includes details and examples of vascular imaging in patients presenting with acute ischaemia and those attending with a presumed transient ischaemic attack (
TIA). The technique is identical and many of the imaging findings are similar. In all cases vascular imaging is required as soon as possible after the event. The risk of further events is highest soon after the initial event. Treatment for the underlying vascular cause should be performed as soon as possible to have maximal future protective effect.
In the past, imaging has been concentrated upon an assessment of the common carotid bifurcation. This is due to the data from the European and American studies comparing medical and surgical treatment of carotid territory stroke in the
ECST and
NASCET trials. In these, digital subtraction angiography (
DSA) was the only vascular imaging used.
The advent of
MDCT, and especially 64-slice
CT, now offers a new and exciting method of assessing the entire cerebral vasculature, from arch to Circle of Willis in only 6 seconds. This is also important as reanalysis of the earlier trials shows that aortic disease can be responsible for ongoing ischaemic events. Posterior circulation ischaemia, increasingly recognized as a cause of stroke in young adults, was largely ignored in previous studies. Doppler ultrasound, a commonly used screening examination, covers little of the necessary vessels, and is of limited value.
MDCTA provides a new solution to all these imaging requirements.
Good vascular imaging can be achieved with a 4-slice
MDCT scanner. The acquisition with a 64-slice scanner provides a significant improvement in the imaging because of improved temporal resolution and relative lack of jugular venous superimposition. With a slice thickness of 0.65 mm compared to 1.25 mm with the 4-slice scanner, the spatial resolution is also markedly improved. All scans are triggered from the contrast density in the ascending aorta with the faster 64-slice scanner. With the 4-slice system the scan was triggered from the pulmonary artery in patients under 50 years to ensure the slower scanner did not miss the contrast bolus. The number of technical failures with 64-slice systems is very low.
It is now possible to confirm or exclude vascular disease in any vessel supplying the brain. The time for an experienced radiologist to perform this total assessment is 15-20 minutes which compares favourably with the 30 minutes required for Doppler ultrasound assessment. The issue of radiation dose is not relevant in patients in the age group usually affected. Our dose for
MDCTA is actually just a little more than that of our helical head protocol. It is necessary to ensure secure intravenous cannulation to avoid potentially hazardous contrast extravasation.
Previously venous reflux and in-flow contrast venous contamination were problematic and could mask pathology at the root of the neck. This is largely resolved by using smaller volumes of contrast, 60 ml compared to 100 ml, and following that with a large bolus of saline. The right arm is preferred for venous access to minimize artefact from incoming contrast. If the left arm is used, beam hardening artefact from contrast remaining the brachiocephalic vein, which passes anterior to the origins of the great vessels, can destroy details of the vessel origins or cause artefactual stenoses.
Reconstruction and reformation
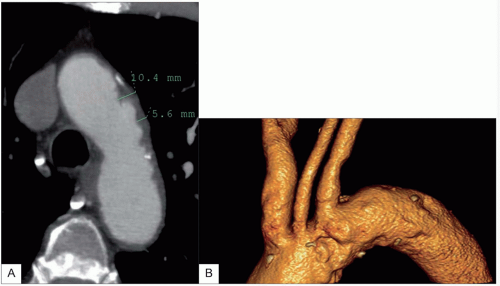
VR3D images provides a rapid overview of the vessels, but base image or
MPR review is necessary to measure accurately the degree of stenosis either as a percentage of diameter or, more accurately, the area of the carotid artery.
Pathology and illustrations
Vascular
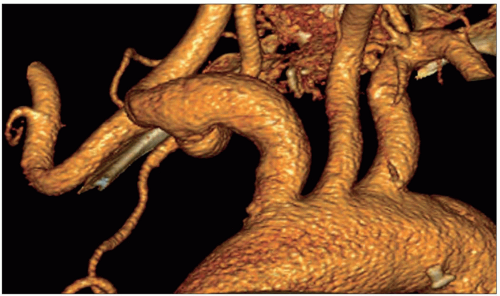
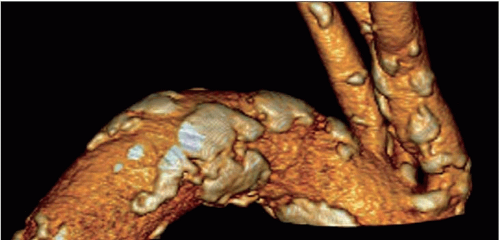
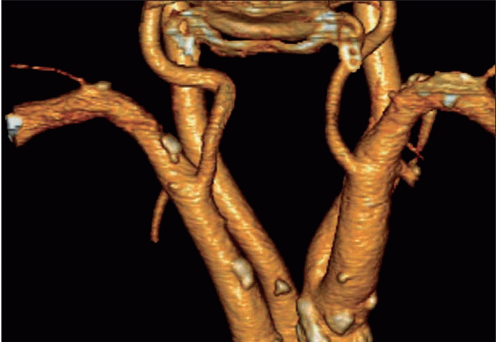
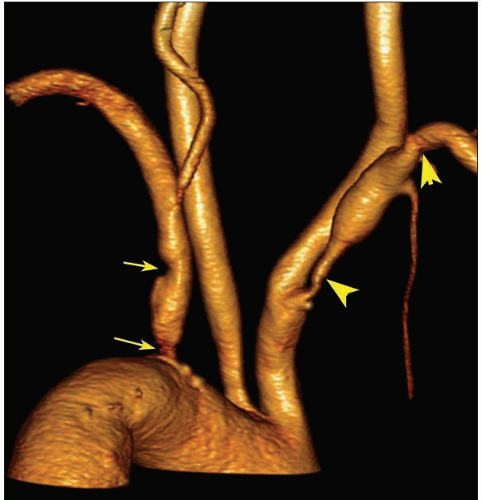
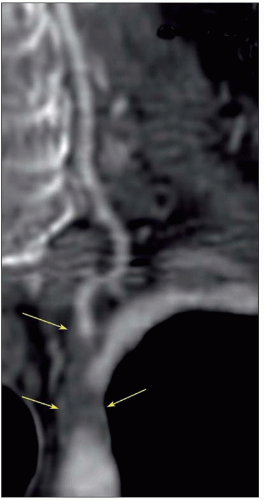
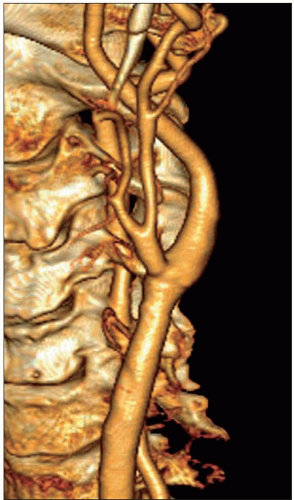
Atheroma is by far the commonest focal causative pathology and is illustrated at many sites.
Dissection:
Vasculitis:
Cardiac
Arhythmias, e.g. atrial fibrillation
Right to left shunt, e.g patent foramen ovale
Tumour, e.g. atrial myxoma


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access