Vascular Malformations of the Brain
Key Points
Most procedural problems during embolization of brain arteriovenous malformations (AVMs) occur at the time of microcatheter withdrawal. Intensive management of the patient following the procedure is also essential to avoid complications related to sudden adjustment of flow in the setting of chronic adaptations of the autoregulatory curve.
Mechanisms of gene regulation and angiogenesis that are responsible for the dynamic nature of brain AVMs over time likely have much in common with those found in other hypervascular conditions such as tumors, dural vascular disease, and chronic occlusive Moya-Moya disease.
The conventional description of vascular malformations of the intracranial blood vessels begins with McCormick from 1966 (1,2) and describes four distinct categories:
Capillary telangiectasis
Cavernous malformation
AVM malformation
Anomaly of venous drainage
While this classification is still reasonably valid for most clinical purposes, an understanding is emerging that thinking about vascular malformations in this categorical way is a blinkered perspective. Many malformations do not fit into one exclusive category raising the question of what the mechanisms of pathogenesis for these lesions might be and how they might impact the treatment of vascular malformations.
It is generally accepted without much controversy that in the case of dural AVMs or fistulas, a preceding venous abnormality such as venous sinus thrombosis or venous hypertension frequently plays a role in the opening of AVM shunts and recruitment of vessels into the developing lesion (3). Since the 1970s cases of dural sinus thrombosis or head trauma leading to subsequent development of dural AVMs have been recognized (4,5). Patients with dural AVMs are more commonly found to have thrombogenic risk factors than the general population, such as factor V Leiden, protein S deficiency, antithrombin III deficiency, and the G20210A prothrombin mutation (6,7,8,9,10,11,12), supporting the contention that venous thrombotic states likely precede a significant number or most cases of dAVMs. It is understandable, therefore, that endovascular obliteration of the venous side of a dural AVM is considered to be an obligatory component of the endovascular cure of a dural AVM, whether the treatment is transarterial or transvenous (see Chapter 20). Similarly, in facial or syndromic AVMs of the extremities, treatment strategies for endovascular obliteration or sclerosis of vascular lesions has come more and more to emphasize the need to gain total control of the lesion by dwelling on obliteration of the venous side (13). The conventional wisdom hitherto with reference to pial AVMs of the brain has been that the fragility and propensity to rupture of the cortical veins would preclude any such primary emphasis in the endovascular treatment of AVMs. This concern is still realistic. However, veins likely play a big role in the genesis of AVMs in the first place and probably have a big influence on factors favoring recanalization or growth of the AVM (see below).
Telangiectasis (Capillary Angiomas)
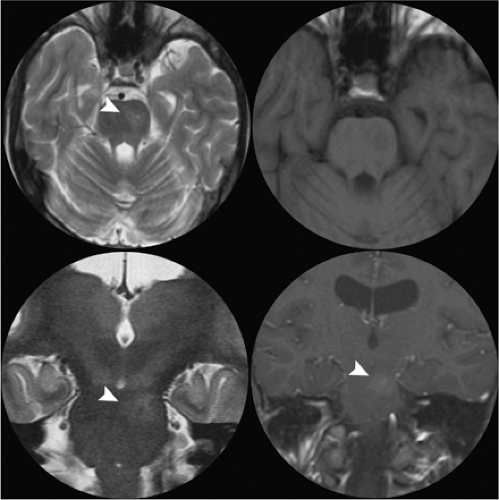
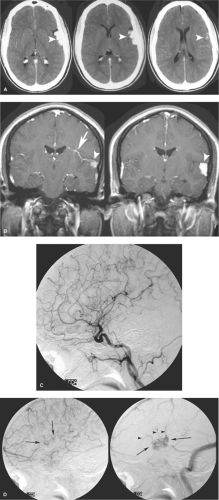
These are common, incidental findings at autopsy, found particularly in the pons. They cannot be seen by angiography and are considered, with cavernous malformations, among the angiographically occult vascular malformations. They consist of ill-defined areas of thin-walled capillaries without smooth muscle or elastic fibers. Normal brain tissue may be found within the interstices of these areas. When saccular dilatations and surrounding gliosis with abundant mineralization are present, pathologic differentiation from cavernous malformations can be difficult (1). However, they are usually not associated with evidence of surrounding gliosis or pigment deposition. Their exact borders can be difficult to discern, even microscopically (14). Occasional reports of symptomatic capillary telangiectasis have been published (15,16), but most studies represent these lesions as incidental curiosities (Fig. 19-1). Hemorrhage in the setting of a capillary telangiectasis is more likely due to the co-existence of a developmental venous anomaly and/or features of a cavernous malformation (17,18).
Cavernous Malformations of the Brain
Cavernous malformations of the brain are described as hamartomatous lesions that are characterized by the presence of sinusoidal, thin-walled vessels. They are usually not seen angiographically unless they are extremely large. They have a more circumscribed or defined border than that seen in capillary telangiectasis and are unlikely to have intervening normal brain tissue (1). The recognition of de novo appearance of cavernous malformations in the brain and the dynamic, changing quality of the disease, particularly in familial forms, has brought an understanding that these lesions are not classified easily as hamartomatous or static (19,20). The gross appearance has been likened to a cluster of mulberries.
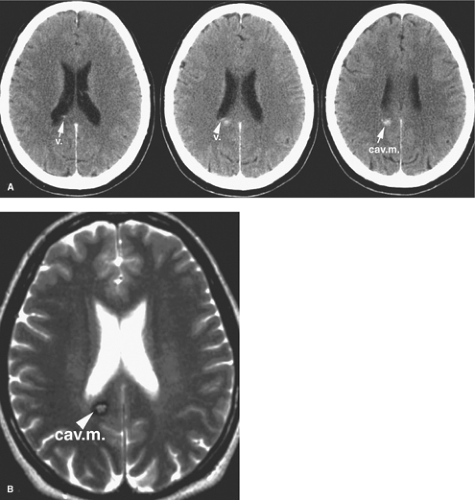
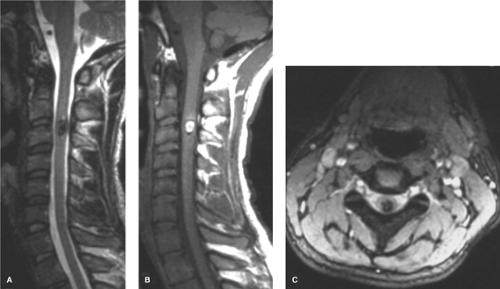
Cavernous malformations have a prevalence of less than 1% in the general population and account for approximately 5% to 16% of vascular malformations of the nervous system (21,22). They can be found anywhere in the brain (Figs. 19-2–19-4) and may also occur in the spinal cord (Fig. 19-5), dura, optic nerve, optic chiasm, and pineal gland. Histologically, they demonstrate a simple endothelial lining and a thin fibrous adventitia devoid of elastin, smooth muscle, or other elements characteristic of mature vascular walls. Although the absence of intervening normal brain tissue is one of the hallmarks of a cavernous malformation, occasionally satellite lesions may give a more complex appearance to their apparent configuration. Foci of maturing thrombosis, hyalinization, calcification, cysts, and cholesterol granules are characteristic, with an absence of large afferent or efferent vessels and of AVM shunting. Surrounding gliosis with ferritin and hemosiderin deposition from repeated hemorrhages or calcification from repeated episodes of thrombosis give the appearance of encapsulation and contribute to the typical MRI ring appearance.
Coexistence of a cavernous malformation with an adjacent anomaly of venous drainage is common, particularly infratentorially, seen in 24% to 86% of lesions (23,24,25). Cavernous malformations associated with a venous malformation can have a more aggressive clinical course (20). The possibility of an associated venous anomaly is of particular concern when surgery is planned, as it is important not to disturb the pattern of venous flow in the associated anomaly (26,27).
Cavernous malformations are multiple in approximately 33% of sporadic cases and in up to 73% of familial cases (19). Familial cases may also demonstrate an increase in the number of detectable lesions when followed over time. De novo genesis of cavernous malformations has been seen in
association with capillary telangiectasis from which they may evolve (28), with venous anomalies (26), at sites of stereotactic biopsy (29), and in patients who have undergone radiation therapy (30).
association with capillary telangiectasis from which they may evolve (28), with venous anomalies (26), at sites of stereotactic biopsy (29), and in patients who have undergone radiation therapy (30).
The natural history of cavernous malformations before they become symptomatic is difficult to evaluate. The best predictor for bleeding in a particular lesion is a history of previous bleeding (31). Asymptomatic lesions that have not previously hemorrhaged have an annual hemorrhage rate of less than 1% per year (32,33). For those lesions with previous bleeding, the annual figure for rebleeding may be as high as 4.5% (34), with a cumulative risk as high as 20% to 80% over a period of weeks to years. Patients who present with nonspecific neurologic symptoms have a risk of developing seizures of approximately 1.5% per year (32).
Cavernous malformations that become symptomatic present with hemorrhage, seizure, or other focal neurologic deficit. Seizures may be simple, complex partial, or generalized, and when found in the temporal lobes may be medically difficult to manage. Considering their size and location, cavernous malformations are considerably more epileptogenic than are AVMs, possibly a reflection of the reactive gliosis and iron deposition, which occur in the periphery of cavernous malformations (34,35).
In addition to seizures, bleeding is a common complication of cavernous malformations. A distinction is drawn between the more common, slow, repeated microhemorrhages associated with cavernous malformations and overt or major hemorrhage, which is reported in 8% to 37% of symptomatic lesions. Significant hemorrhage from a cavernous malformation can be identified by CT or MRI with blood outside the confines of the hemosiderin ring, in the subarachnoid space, or with clinical evidence of apoplectic hemorrhage (31). Hemorrhage may rarely be fulminant and life-threatening but is usually milder than that associated with AVMs. Hemorrhage from a cavernous malformation is usually associated with onset of severe headache and clinical signs and has a stepwise pattern of worsening deficits according to the location of hemorrhage. Lesions in the brainstem are most likely to present with severe cranial nerve or long tract deficits, as they are particularly likely to affect multiple pathways. Intraventricular cavernous malformations can cause obstructive hydrocephalus and hypothalamic symptoms (36,37). Cerebellar lesions present with nausea, vomiting, nystagmus, ataxia, and diplopia.
The clinical behavior of particular cavernous malformations changes with time in response to the effects of cumulative intralesional hemorrhage, thrombosis, and mineralization. Age is a significant factor in the natural history of these lesions, with most patients presenting in the second to fourth decades. Children who are symptomatic with such lesions have a greater likelihood of significant hemorrhage and of acute neurologic deficits than young male adults who present with seizures. Female adults and children tend to demonstrate significant hemorrhage and neurologic deficits (31,33,35,38), and the clinical course of cavernous malformations in women is adversely affected during pregnancy (20,21). Hormonal factors may influence the behavior of
cavernous malformations because they are more likely to become hemorrhagic in females. A more aggressive clinical course has been reported also for patients with familial forms of the disease, patients with multiple lesions, patients with previous whole brain or stereotactic radiotherapy, and patients with lesions with an intraventricular location or an associated venous anomaly.
cavernous malformations because they are more likely to become hemorrhagic in females. A more aggressive clinical course has been reported also for patients with familial forms of the disease, patients with multiple lesions, patients with previous whole brain or stereotactic radiotherapy, and patients with lesions with an intraventricular location or an associated venous anomaly.
Anomalies of Venous Drainage
The venous angioma, venous malformation, or anomaly of venous drainage represents, at a basic level, an apparently innocuous developmental aberration of venous arborization. On a more abstract level, these commonplace findings may provide a useful insight into the mechanism of development of brain AVMs.
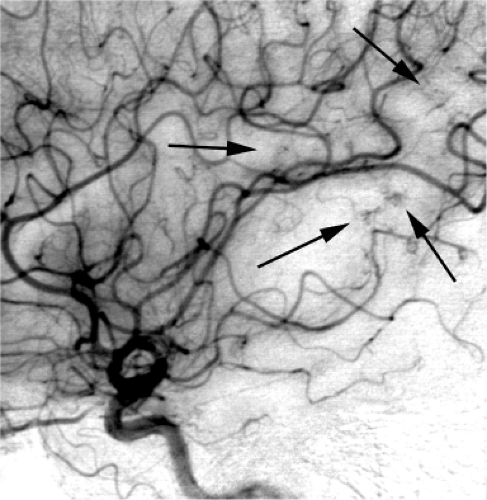
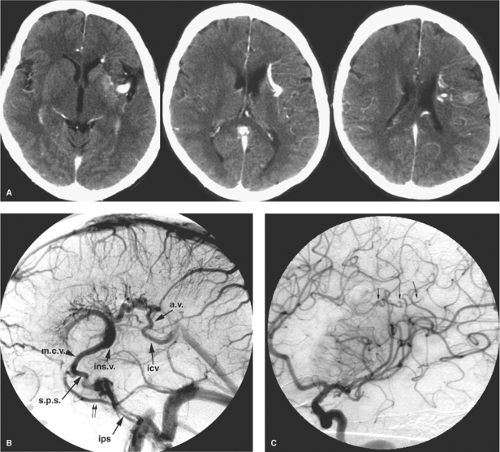
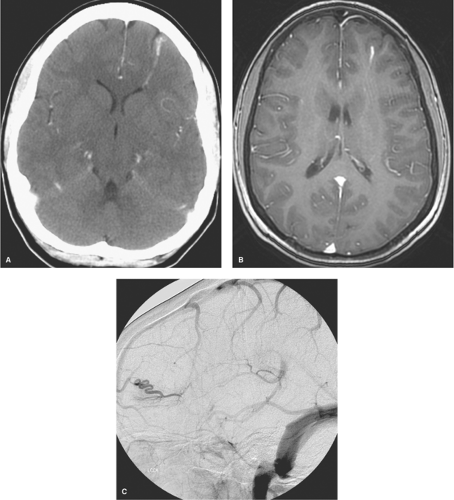
Venous anomalies are commonly found at autopsy, identified in 2.5% of a large autopsy series (2,39). Surrounded by normal neural parenchyma, they consist in a converging pattern of abnormally prominent medullary veins draining to a single trunk (Figs. 19-6 and 19-7). Frequently disposed in a radial or star pattern, the appearance of these prominent veins is likened to the “caput medusae.” Occasional instances of this lesion are seen where the draining vein has a particularly distended and indolent aspect, earning the qualification of an “intracerebral varix” (40,41). Although rare, an intra-axial varix can constitute a diagnostic hazard, as it can resemble a cystic mass on CT or MRI (42) (Fig. 19-8).
Usually, these anomalies appear small on axial imaging, with vessel components extending over an area measuring 2 to 3 cm or less in maximal dimensions. Although they are so small, such dimensions amplify into considerably larger venous territories paying tribute to a venous anomaly. The significance of this observation is evident in the consequences of extensive venous infarction and hemorrhage which can follow surgical excision of these lesions. Therefore, they are now conceptualized as developmental anomalies. They are thought to involve little if any innate risk and do not warrant any specific treatment in most circumstances, with an innate risk of hemorrhage estimated to be less than 1% per year (43,44,45,46).
The clinical significance of venous anomalies is first to recognize them as distinct from AVMs and to treat conservatively. Second, the anomaly of venous drainage has a propensity to occur in association with more symptomatic lesions, particularly with cavernous malformations (47). The latter agent is thought responsible when venous anomalies are seen in close proximity to small intraparenchymal areas of hemorrhage. If treated surgically, it is important to preserve the integrity of the venous components of the anomaly and to confine the resection procedure to the cavernous malformation.
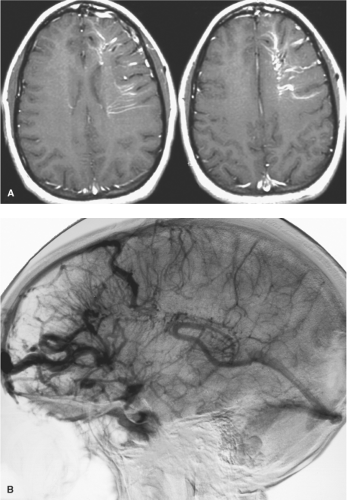
Venous anomalies are frequently associated with aberrations or deficiencies of adjacent pial veins and dural sinuses, similar to those seen with AVMs of the brain. Mullan et al. (48) have suggested that cerebral venous anomalies and AVMs may have a related mechanism of genesis, arguing cogently that an AVM represents a fistulization of a venous anomaly (Fig. 19-4). Time and a greater understanding of cell signaling have supported the prescience of this insight. Venous hypertension within the territory of a venous anomaly can occasionally be suspected when parenchymal signal changes are seen on FLAIR and T2 imaging, presumably representing the first stages of complications of venous thrombosis or compromise of venous flow. Analogous to the pathophysiology of dural AVMs following venous sinus thrombosis, there is a persuasive body of evidence that suggests that brain AVMs and cavernous malformations may be generated as de novo lesions in a similar way (3). Gene expression of vascular endothelial growth factor (VEGF), and angiogenic factors such as integrin receptors, matrix metalloproteinases (particularly MMP-9), angiopoietin, and basic fibroblast growth factor (bFGF) can be altered in a sustained manner by physiologic changes in blood flow, shear stress, and venous pressure, leading in the long term to anatomic and
vascular alterations that become clinically recognized as one type of malformation or another. Depending on the nature of the initial insult, the particular genetic profile of the patient, and the timing in life of the event, complications of venous pathology could lead to expression of an AVM, a cavernous malformation, or a mixed type of lesion. Pial AVMs, for instance, are thought to have a slightly more mature endothelial profile with higher ratio of laminin to fibronectin and thus a greater stability of the basement membrane compared with the more friable and immature composition of cavernous malformations where fibronectin predominates and laminin is deficient (49,50,51,52).
vascular alterations that become clinically recognized as one type of malformation or another. Depending on the nature of the initial insult, the particular genetic profile of the patient, and the timing in life of the event, complications of venous pathology could lead to expression of an AVM, a cavernous malformation, or a mixed type of lesion. Pial AVMs, for instance, are thought to have a slightly more mature endothelial profile with higher ratio of laminin to fibronectin and thus a greater stability of the basement membrane compared with the more friable and immature composition of cavernous malformations where fibronectin predominates and laminin is deficient (49,50,51,52).
Arteriovenous Malformations of the Brain
AVM malformations of the brain are vascular lesions in which an abnormal tangle or nidus of vessels permits pathologic shunting of blood flow from the arterial to the venous tree without an intervening capillary bed (Figs. 19-9 and 19-10). They are frequently described as congenital, but this is questionable. Incidental brain AVMs are rarely seen in children, even with the endemic use of CT scanning currently in vogue for every minor trauma. Furthermore, an unusual
form of AVM can be seen in children, specifically a pial AV fistula, which is very rare in adults, raising the question of whether a single vessel AVF would mature over the years and grow with the child into a more complex nidal type of AVM in adulthood (53,54,55) (Figs. 19-11 and 19-12).
form of AVM can be seen in children, specifically a pial AV fistula, which is very rare in adults, raising the question of whether a single vessel AVF would mature over the years and grow with the child into a more complex nidal type of AVM in adulthood (53,54,55) (Figs. 19-11 and 19-12).
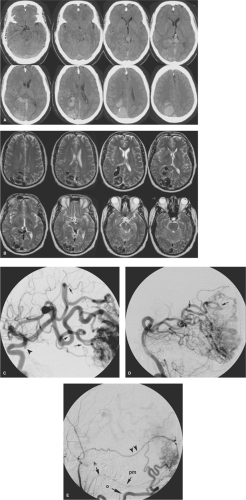 Figure 19-9. (A–B) Large brain AVM. An untreated brain AVM of the right occipital lobe illustrates the gamut of imaging findings typically associated with larger, easy-to-identify lesions. The contrast-enhanced CT (A) and T2-weighted MRI (B) demonstrate the massive distension of veins within the parenchyma and ventricle associated with this lesion. There is gliosis and involution of adjacent parenchymal structures, perhaps in response to “steal phenomenon,” that is, relative ischemia due to the sump of the AVM. Previous hemorrhage could account for this finding as well but was not known in this patient. Venous pressure is so great that there is distortion and mass effect on the brainstem and adjacent structures. The right internal carotid artery angiogram (C) illustrates the markedly overgrown and slightly dysplastic feeding vessels (focal dysplasia marked with arrows in C) of the middle cerebral artery. A small, flow-related aneurysm on the right posterior communicating artery is present (arrowhead in C). The left vertebral artery injection (D) shows areas of focal dysplasia, but some of the feeding vessels appear to have a less direct route of access to the AVM than was the case with the middle cerebral artery. This is probably in part a reflection of “angiomatous change” in the branches of the right posterior cerebral artery, where arteries perfusing normal adjacent tissue undergo hyperplasia in response to the demand for flow from the AVM. The right external carotid artery injection (E) shows a component of dural supply to the AVM as well. This is mediated via an enlarged middle meningeal artery (double arrowheads), the occipital artery (o), and the posterior meningeal artery (pm), which derives from the hypoglossal branch (h) of the ascending pharyngeal artery.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|