Vascular Malformations of the Spine
Key Points
All of the available sources of supply to the anterior spinal artery may not declare themselves readily on the initial angiographic evaluation. Care should always be exercised when using particulate or liquid agents near the spinal cord, lest an unsuspected pedicle to the anterior spinal artery be present.
Manifestations of spinal venous hypertension may be very subtle, particularly on nonenhanced MRI examinations.
Introduction
Spinal cord vascular malformations represent about 2% to 3% of spinal masses and typically present with myelopathic symptoms related to compromise of long tract function (bowel and bladder dysfunctions, motor and sensory losses) but can also present with back pain, radicular symptoms, subarachnoid or intraspinal hemorrhage, or spinal deformity.
Spinal arteriovenous malformations are rare. With the exception of spinal dural arteriovenous malformations, there are probably almost as many publications and classification systems for spinal vascular disease in the literature as there are patients with these disorders. The most helpful way to understand these lesions is by their location with reference to the dura and the spinal cord. Secondarily, they can be categorized into simple fistulous lesions (AVF) or multivessel lesions (AVM). Intramedullary AVMs are classified into juvenile type (whole cross-sectional area of the cord involved) or glomus type (a more restricted pea-like configuration). Pial fistulas are usually on the surface of the cord or just below and are sometimes graded according to the degree or absence of ectasia of the feeding arteries.
Spinal AVMs can alternatively be classified from a genetic point of view:
Genetic hereditary lesions of the vascular germinal cells, such as seen in hereditary hemorrhagic telangiectasia.
Genetic nonhereditary lesions with metameric expression, such as Cobb syndrome where patients have multiple shunts and malformations of the spine, muscles, paraspinal and cutaneous tissues.
Dural arteriovenous fistulas, on the other hand, are tiny, slowly flowing lesions, which with rare exceptions represent an acquired disease of later adulthood (>40 years). They are supplied by dural arteries and typically affect the lower spine and lower extremities. They cause a more progressive slow decline compared with intramedullary vascular malformations. They do not typically cause subarachnoid hemorrhage.
Intradural Spinal Arteriovenous Malformations
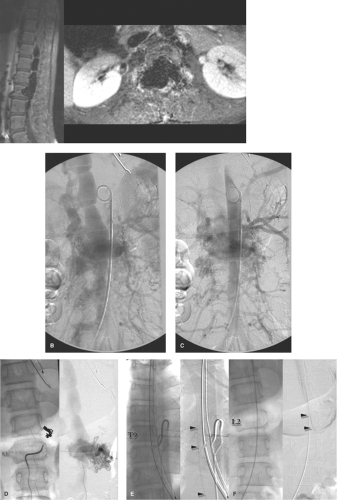
Intradural, intramedullary spinal arteriovenous malformations occur most commonly in the cervical or thoracic cord and are classified into three types (Figs. 21-1–21-5).
Juvenile-type Spinal Arteriovenous Malformations
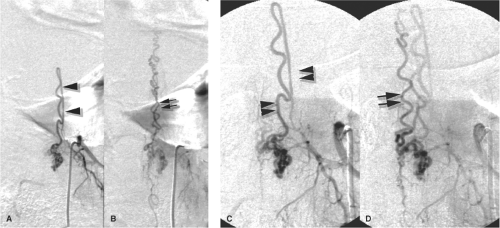
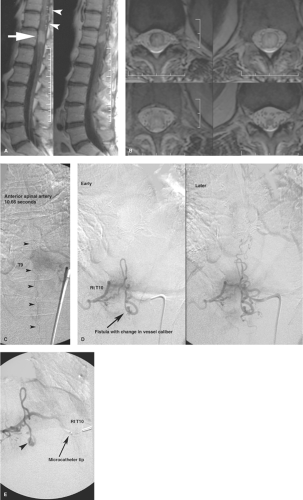
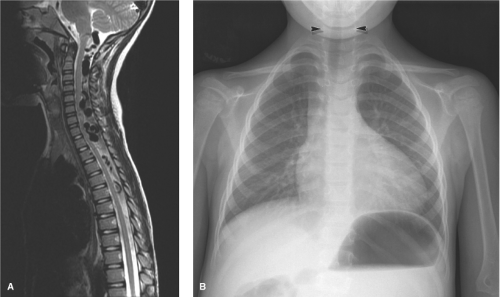
The juvenile type of spinal arteriovenous malformations occupies the entire spinal canal at the involved level(s) and can have normal spinal parenchyma present within the interstices of its components. There may be multiple medullary arterial feeders, most prominently the anterior spinal artery, which, like the arterial feeders to arteriovenous malformations in the brain, may have a dysplastic appearance. Feeding pedicle or intranidal aneurysms may be seen in approximately 20% of intramedullary arteriovenous malformations (3,4), as can venous ectasia or venous aneurysms. A minority of patients show a widened interpedicular distance and may even emit an audible spinal bruit (Fig. 21-6). They can present with gradual or abrupt onset of motor and sensory symptoms related to the level of involvement or with spinal subarachnoid hemorrhage.
Glomus-type Spinal Arteriovenous Malformations
Glomus-type spinal arteriovenous malformations are more compact and defined than the juvenile type. They do not have intermingled normal spinal tissue, are confined to a shorter segment of cord, and are usually fed by a single arterial pedicle. They have a risk of subarachnoid hemorrhage higher than that of other types of intradural vascular malformations, seen in 85% of symptomatic patients (3).
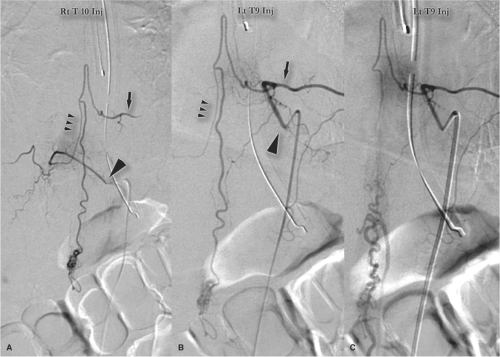
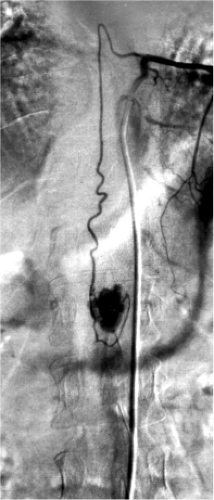
Intramedullary and Perimedullary Arteriovenous Fistulas
Fistulas of the spinal cord may be intramedullary or perimedullary. Flow is usually from an anterior or posterior
spinal artery, which connects directly to a vein without an identifiable nidus. Flow is faster than that seen in dural arteriovenous fistulas. They can present with intramedullary or subarachnoid hemorrhage, but this appears to be a minority of cases. As is the case for dural arteriovenous fistulas, venous hypertension with cord edema appears to be an important pathophysiologic mechanism for symptoms of intradural arteriovenous shunts (5). Aneurysms of the feeding pedicles are not as likely to be present with perimedullary fistulas compared with intramedullary arteriovenous malformations (4).
spinal artery, which connects directly to a vein without an identifiable nidus. Flow is faster than that seen in dural arteriovenous fistulas. They can present with intramedullary or subarachnoid hemorrhage, but this appears to be a minority of cases. As is the case for dural arteriovenous fistulas, venous hypertension with cord edema appears to be an important pathophysiologic mechanism for symptoms of intradural arteriovenous shunts (5). Aneurysms of the feeding pedicles are not as likely to be present with perimedullary fistulas compared with intramedullary arteriovenous malformations (4).
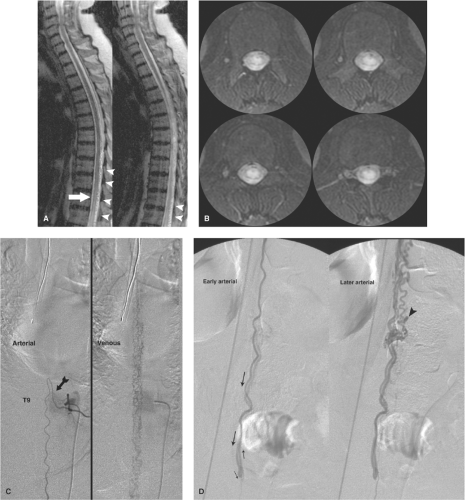
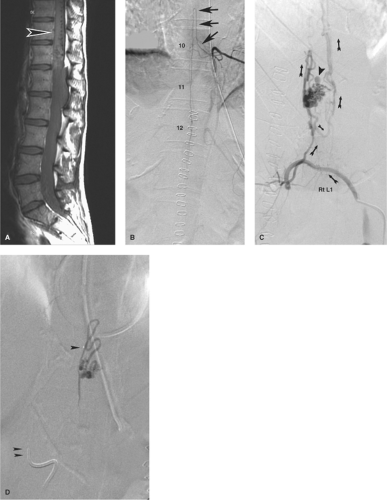 Figure 21-2. (A–D) Intramedullary spinal arteriovenous malformation presenting with intraconal hemorrhage. When this young patient collapsed in a setting of courtroom stress and became paralyzed from the waist down, it seemed to be a textbook example of a hysterical conversion disorder. However, her MRI showed a large intraconal hemorrhage, which was evacuated emergently. A postoperative sagittal MRI (A) revealed a profusion of flow voids on the dorsum of the conus (arrowhead in A), which had not been seen on the preoperative scan. In contrast to that shown in Figure 21-1, the anterior spinal artery at left T10 (arrows in B) has a more normal appearance with no pathologic venous opacification seen. The offending lesion is an arteriovenous malformation of the surface of the cord found on the right L1 injection (C, oblique view with arrows showing sequence of flow). An intranidal aneurysm, which is likely the site of hemorrhage, is noted (arrowhead in C ). The postembolization roadmap view (D) shows the ease with which inadvertent reflux of liquid embolic agent can occur when one is attempting to perfuse the nidus of a lesion. In this instance, there was a presumed safety margin between the microcatheter tip (single arrowhead in D) and the main catheter tip (double arrowhead in D) at the aortic wall, but as a general rule, relying on such landmarks is not foolproof. Reflux of liquid could permeate adjacent segmental arteries through collateral pathways with unintended results. |
Paraspinal and Epidural Arteriovenous Malformations
Paraspinal AVMs are rare lesions that can affect the paravertebral muscles, nerve root foramina, or prevertebral structures (6). They can be seen posttraumatically, but most are considered to be congenital lesions presenting in children with back pain, paraparesis, high-output cardiac failure, or an audible bruit (Fig. 21-7). Most of them seem to exert their functional effect through venous congestion transmitted to the intradural veins.
Hypervascular Tumors Simulating Arteriovenous Malformations
Arteriovenous shunting or early venous opacification can be seen with vascular intra-axial tumors such as glioblastoma multiforme (Figs. 21-8 and 21-9) or hemangioblastoma. Extra-axial vascular tumors, such as hemangiopericytoma and extra-axial or spinal hemangioblastoma, may also give an appearance that could be confused with that of an arteriovenous malformation. Usually, the vascularity of such tumors is easily recognized as subordinate in prominence to other evidence of a mass lesion. Arteriovenous shunting is rarely as fast in a tumor as that seen in arteriovenous malformations. A parenchymal blush characteristically seen in vascular tumors is not seen in arteriovenous malformations.
Endovascular Treatment of Spinal Vascular Malformations
The prognosis for an untreated symptomatic spinal AVM is poor with 36% of patients younger than 40 years progressing to severe impairment of function within 3 years (7). Safe endovascular embolization of spinal vascular lesions hinges on the ability to distinguish between a posterior spinal artery and an anterior spinal artery. Typically an average-sized PSA supplies a small rim of peripheral tissue centripetally in the posterior third of the cord and its loss, inadvertent or otherwise, is thought to be well tolerated due to the proclivity to develop collateral pathways of supply (8). The anterior spinal artery is a different story. Typically it supplies the greater portion of the cross-sectional territory of the spine at any one level due to penetrating branches from the anterior median sulcus, the sulco-commissural branches, which measure between 400 and 100 μm in size (9) tapering to approximately 60 μm as they penetrate the cord parenchyma centrifugally (10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree