Vein of Galen Malformations
Key Points
The age of presentation, gravity of the condition, and difficulty of treatment of vein of Galen malformations are highly linked to the angioarchitectural subtypes of this disorder.
Most intraprocedural complications during treatment of these lesions can be ascribed to the extreme delicacy of the intracranial vessels in neonates and infants who require treatment in early life. Mundane endovascular maneuvers are not as easily tolerated by these thin-walled, distended structures.
The treatment of vein of Galen malformations is among the most difficult of neuroendovascular challenges, requiring intensive collaboration between obstetrical, neonatal intensivist, pediatric cardiology, and anesthesia staff, often beginning with a prenatal diagnosis. The most effective ultimate treatment modality is endovascular closure of the shunting vessels, but the welfare of the patient is best served by deferring this procedure for as long as is safely possible.
Introduction
The first description of a vein of Galen malformation/aneurysm dates to 1937 (1). Fortunately, these are extremely rare lesions, and it has been estimated that a neurosciences center serving a population of 3 million will see a patient with this diagnosis less than once per year (2). This group of lesions is characterized by the midline presence of enlarged arteries in the region of the quadrigeminal cistern shunting into a massively enlarged venous sac, previously thought to represent the vein of Galen. The embryonic origins of this lesion have been clarified by Raybaud and subsequent authors (3) as a shunt that becomes established between 6 and 11 weeks of gestation resulting in persistence of the median vein of the prosencephalon and secondary failure of development of the normal deep venous drainage system. Therefore, in most patients with this disorder the venous drainage pattern, which at a glance can look similar to an enlarged version of the vein of Galen and straight sinus, actually represents a primitive precursor of the usual venous system, with variant channels of drainage through falcine and tentorial sinuses that would have regressed under otherwise normal circumstances. The practical implications of this nice point are two-fold:
The brain does not therefore develop the usual system of deep venous drainage, and alternative venous pathways persist resulting often in enlargement of venous routes on the face, subcutaneous tissues, or scalp.
The presumption that the brain does not drain to the venous sac of the malformation would imply that therapeutic venous occlusion of the sac should be possible without risking venous infarction of the brain (although this presumption is questioned as not entirely reliable in all patients by some authors).
The patterns of arterial feeders to the malformation fall into two categories with significant implications on the clinical presentation of the patient and on the challenges of endovascular treatment.
Mural type of vein of Galen malformations account for about 30% to 40% of lesions and are the better type to deal with in every way (4,5). The number of arterial feeders is limited and usually confined to discrete, enlarged posterior choroidal arteries from the proximal posterior cerebral arteries (Fig. 33-1). They are named according to the discrete nature of the shunt in the wall of the venous sac. The volume of flow is not quite as high as the choroidal type of lesion, and thus angiographic images tend not to be quite so washed out, with better definition and visualization of the arterial feeders and venous sac. These children are less likely to develop immediate problems with cardiac output and tend to present later in childhood—or reach a point of needing treatment later—with macrocephaly, developmental delay, and seizures.
Choroidal type of vein of Galen malformations account for the majority of cases in most series. Their appearance is wilder on the angiogram with extremely rapid flow, markedly sinuous vessels, and involvement of choroidal vessels from the posterior circulation, thalamoperforator branches, and also the distal choroidal branches of the anterior cerebral artery (Fig. 33-2) (6). Occasionally, dural branches from the tentorium or falx can be recruited too, originating from the cavernous segment of the internal carotid artery or external carotid artery.
Clinical Presentation and Management
The near universal practice of prenatal ultrasonography means that these lesions are now sometimes detected in utero with a midline cystic intracranial lesion demonstrating color Doppler flow. Prenatal MRI has the same capacity
and also allows an evaluation of the condition of the rest of the brain. Most prenatals with this disorder tolerate the shunting well due, it is thought, to the extremely low resistance of the placental circulation, meaning that the shunting within the malformation does not become relevant until after birth. Children with large volume shunts, typically those with choroidal-type malformations, tend to become symptomatic within a few days of birth or early in the neonatal period with high-output cardiac failure. With as much as 70% or more of the cardiac output shunting through the lesion, return to the heart is overwhelming and pulmonary hypertension with persistent right-to-left shunting in the heart and ductus arteriosus quickly becomes a dominant management problem. Reduced arterial systemic diastolic pressure, with biphasic reversal of flow in the aorta, results in hypoperfusion of the coronary, renal, and hepatic circulations. Tachypnea and difficulty feeding quickly leads to failure to thrive and eventual multiorgan failure in many patients, with a virtual 100% mortality rate documented in older papers before effective treatment was a possibility (7). The cerebral circulation is particularly vulnerable due to the combination of hypoperfusion from cardiac failure, physiologic arterial steal due to the size of the shunts, and elevated venous pressure secondary to the effects on venous outflow. Early changes of parenchymal calcification progressing to more advanced “brain-melting” syndrome likely constitute an effective deterrent to treatment and most of the time indicate a poor prognosis.
and also allows an evaluation of the condition of the rest of the brain. Most prenatals with this disorder tolerate the shunting well due, it is thought, to the extremely low resistance of the placental circulation, meaning that the shunting within the malformation does not become relevant until after birth. Children with large volume shunts, typically those with choroidal-type malformations, tend to become symptomatic within a few days of birth or early in the neonatal period with high-output cardiac failure. With as much as 70% or more of the cardiac output shunting through the lesion, return to the heart is overwhelming and pulmonary hypertension with persistent right-to-left shunting in the heart and ductus arteriosus quickly becomes a dominant management problem. Reduced arterial systemic diastolic pressure, with biphasic reversal of flow in the aorta, results in hypoperfusion of the coronary, renal, and hepatic circulations. Tachypnea and difficulty feeding quickly leads to failure to thrive and eventual multiorgan failure in many patients, with a virtual 100% mortality rate documented in older papers before effective treatment was a possibility (7). The cerebral circulation is particularly vulnerable due to the combination of hypoperfusion from cardiac failure, physiologic arterial steal due to the size of the shunts, and elevated venous pressure secondary to the effects on venous outflow. Early changes of parenchymal calcification progressing to more advanced “brain-melting” syndrome likely constitute an effective deterrent to treatment and most of the time indicate a poor prognosis.
Patients with milder type of choroidal malformations or mural-type malformations are less severely affected in the neonatal period and may remain asymptomatic or be managed medically until they have grown older (>6 months) at which time they can tolerate an endovascular procedure more safely. Frequent clinical and MRI evaluations during this time of waiting are advised, with any evidence of progression of macrocephaly or developmental delay being
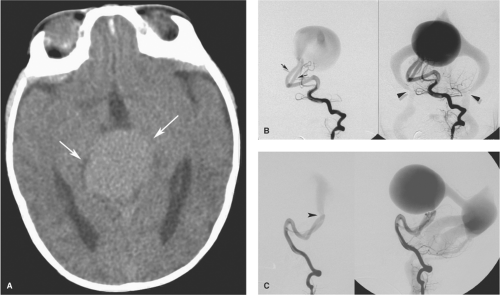
taken as a trigger to initiate endovascular treatment (8). Typically mural-type malformations present with macrocephaly, hydrocephalus, seizures, or developmental delay, with cardiac failure being a less prominent feature as the age of presentation increases. Occasionally a vein of Galen malformation may escape detection completely during childhood and may present in adulthood, typically with a subarachnoid bleed or macrocrania (Figs. 33-3 and 33-4).
taken as a trigger to initiate endovascular treatment (8). Typically mural-type malformations present with macrocephaly, hydrocephalus, seizures, or developmental delay, with cardiac failure being a less prominent feature as the age of presentation increases. Occasionally a vein of Galen malformation may escape detection completely during childhood and may present in adulthood, typically with a subarachnoid bleed or macrocrania (Figs. 33-3 and 33-4).
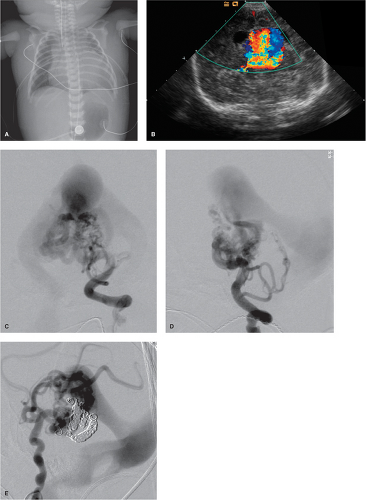 Figure 33-2. (A–E) Choroidal-type vein of Galen aneurysm in a premature infant. This infant was born by Caesarean section at 37 weeks of gestation with a prenatal diagnosis of vein of Galen aneurysm and suspected hydrops fetalis. The postnatal condition was frail with high-output cardiac failure and low renal output. The chest film (A) shows cardiomegaly, soft tissue edema from hydrops, and bilateral chest tubes. The postnatal cranial ultrasound (B) confirmed the diagnosis of vein of Galen aneurysm. The patient showed a faintly optimistic response to an infusion of prostaglandin E aimed at maintaining patency of the ductus arteriosus. This and the interpretation of the cranial ultrasound as showing multiple discrete jets of flow within the sac, implying that the lesion might be a mural type, prompted an override of initial reluctance to attempt treatment in this case. The AP (C) and lateral (D) views of the left vertebral artery injection showed, however, that this was a choroidal type of lesion with a more complex morphology than that illustrated in Figure 33-1. Multiple choroidal feeders are involved with enlargement and tortuosity of circumflex and perforator vessels at the level of the mesencephalon. Multiple feeders from the anterior circulation were subsequently seen as well from the right internal carotid artery during a second procedure (E). Sadly, despite a promising response to the first embolization, the patient did not survive the second due to an intraventricular bleed.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|