10 Deep vein thrombosis (DVT) most commonly involves the deep veins in the lower extremities.1–3 Thrombosis confined to the calf veins or distal DVT usually remains confined to the deep veins distal to the popliteal vein and has no serious consequences.4 However, approximately 20% extend into the popliteal vein and become proximal.4 Proximal vein thrombosis involves the popliteal, femoral, and iliac veins and is the most common source of pulmonary embolism (PE).5 Less commonly, the deep veins in the upper extremity may become thrombosed particularly in patients with indwelling vascular devices.6–8 Thrombosis may occur in more unusual locations, such as the cerebral venous sinus or the venous system in the splanchnic bed.6 Superficial venous thrombosis, commonly referred to as thrombophlebitis, is a relatively benign disorder except where the thrombus may extend into the deep venous system in the groin or at the level of the knee.9 Pulmonary embolism most commonly originates in the deep venous system of the legs.5 Other less common sources include the deep pelvic veins; the renal veins or inferior vena cava; the right side of the heart, particularly in patients with cardiomyopathy or ischemic heart disease; and rarely from the axillary veins.1 Up to 70% of patients with PEs have evidence of DVT, and more than 50% of patients presenting with a proximal DVT already have PE. DVT and PE are referred to collectively as venous thromboembolism (VTE).9 The incidence of VTE is difficult to ascertain, as many patients are asymptomatic. This is particularly true in patients in the postoperative period or in other hospitalized patients, because as many as 50% of these cases of VTE may be silent.1 The increasing use of more sensitive multidetector computed tomography (CT) scanners has markedly increased the incidence of PE, many of which cases are asymptomatic or incidental, and as the scanners have become more sensitive, more subsegmental PEs are being reported.10,11 However, there has been no change in the number of central or fatal PEs over this same time period. The management of these unexpected PEs is still controversial and, except for the small subsegmental emboli, anticoagulant treatment is recommended. The annual incidence of VTE is estimated to be approximately 117 cases per 100,000 persons.12 Estimates in the United States place the incidence of VTE somewhere between 350,000 and 600,000 cases per year.13 Hospital discharge data in the United States for the year 2003 show that there were over 38,000,000 hospital discharges, with 12,000,000 of these patients on both medical and surgical wards being at risk for VTE based on the American College of Chest Physicians (ACCP) guidelines.14 Effective prophylaxis against VTE is now available for most high-risk patients (see also Chapter 11).15 Most fatal PEs occur suddenly and without warning, demonstrating the importance of prevention as the most critical step for reducing death from PE. Furthermore, the prevention of death and morbidity from VTE is more cost-effective than treating the established disease.16 Numerous national and international guidelines are available for the prevention of VTE such as those produced regularly by the ACCP.15 Clearly the most feared complication in patients with VTE is fatal PE.17–19 Patients with massive PE have an increase in pulmonary vascular resistance from the thrombus obstruction as well as pulmonary vascular constriction.18 This leads to increased pressure in the right side of the heart and septal shift toward the left ventricle, which leads to arterial hypotension, cardiogenic shock, and cardiac arrest unless the obstruction can be lessened by thrombolysis or by mechanical means. The spectrum of PE ranges from asymptomatic patients with incidental PE to patients with submassive PE who may have hypotension and right ventricular dysfunction but who will survive if further embolism can be prevented with anticoagulation.18,19 Also many patients with PE have evidence of repeat small embolism prior to the larger pulmonary embolus, which leads to the diagnosis.18 Rarely, patients who have DVT and a patent foramen ovale may develop paradoxical embolism of thrombotic material leading to stroke.20,21 The postthrombotic syndrome (PTS) results from venous valve dysfunction with or without persistent proximal vein obstruction leading to venous hypertension. This can result in the redirection of blood flow from the deep venous system to the superficial veins with increased edema, impaired circulation, and ultimately venous ulceration.22,23 The PTS, therefore, presents as a spectrum that includes lower extremity edema that is worse by the end of the day, redness and hyperpigmentation, and venous ulceration. PTS develops in up to 30% of patients after an initial episode of proximal DVT.22,23 The development of recurrent iliofemoral thrombosis in the same leg increases the risk of developing PTS, as does inadequate anticoagulation early in the treatment.24,25 The use of graduated compression stockings has been shown to significantly decrease the incidence of PTS, particularly the more severe forms, if the stockings are applied shortly after the development of proximal DVT and are used consistently for up to 2 years.26,27 Chronic thromboembolic pulmonary hypertension (CTPH) may develop in 0.5 to 1.5% of patients presenting with an initial PE, with some estimates being even higher.28–30 CTPH may not develop for months or even years following the initial PE. There is a correlation of the development of CTPH with a degree of vascular obstruction, and there is some evidence that impaired fibrinolysis or altered fibrinogen may contribute to the development of the syndrome.31 Without surgical correction there is a high incidence of both morbidity and mortality.28–30 Venous thrombi are composed mainly of fibrin and red blood cells with variable numbers of leukocytes and platelets. Thrombosis usually begins in the valve pockets in the distal veins and propagate proximally.32 The thrombogenic stimuli as identified by Virchow still apply: stasis of blood, activation of blood coagulation, and damage to vessel walls.31 The protective mechanisms include lysis by fibrinolytic enzymes derived from plasma and endothelial cells, clearance of activated coagulation factors by mononuclear phagocytes in the liver, and inactivation of activated coagulation factors by circulating inhibitors such as antithrombin and activated protein C.32 Several acquired and inherited risk factors for VTE have been identified.33,34 Patients who develop VTE at a younger age, multiple family members with VTE, idiopathic VTE, recurring VTE, or a history of multiple spontaneous abortions should be evaluated for acquired or inherited coagulopathies, the so-called thrombophilias.35 The most common of these are factor V Leiden, the prothrombin mutant, and the acquired antiphospholipid antibody syndrome.36 Patients with antithrombin deficiency or those with multiple forms of thrombophilia are at highest risk for thrombosis. The use of oral contraceptive pills or pregnancy may be the first indication of a predisposition for thrombosis in patients with thrombophilia.35,37 Patients with idiopathic or unprovoked VTE or with ongoing risk factors such as cancer or prolonged immobility are at the greatest risk for recurrent VTE.38–40 Various strategies for risk assessment for recurrent VTE incorporating D-dimer testing have been developed (e.g., REVERSE Study or DASH), which include factors such as PTS, age, sex, obesity, and the sex hormone–associated VTE.41–43 These factors have been shown to have predictive value for recurrent VTE, but have not as yet become part of clinical practice. Risk factors for upper extremity DVT include younger age, male sex, smoking, and indwelling intravascular devices. Indeed, indwelling intravascular devices account for 80% of upper extremity DVT.44,45 Patients with brain tumors are at high risk for VTE.46–54 Risk factors include malignant tumor, advanced age, longer duration of surgery, and paresis. In one study, the risk of clinically diagnosed VTE within 30 days of craniotomy was 3.9% for all patients and was especially high for patients undergoing craniotomy for primary malignancy (7.5%) and for metastasis (19%).47 In addition to surgery, radiotherapy and chemotherapy contribute to the risk of VTE in brain tumor patients.52–54 The overall risk of VTE following spinal surgery is relatively low, but higher risk factors include a combined anterior-posterior approach, multiple operative levels, and patient-related factors such as older age, prior VTE, and malignancy.55,56 A population-based retrospective analysis of discharges from California hospitals found the risk of symptomatic VTE within 90 days of surgery to be 0.5% among patients who underwent spinal surgery for a nonmalignant disease, whereas the risk of VTE was 2.0% for patients who had spinal surgery for malignant disease.48 The ACCP classifies spinal surgery as low risk for most patients with nonmalignant disease and moderate for patients with malignancy.50 Trauma patients pose a high risk for VTE, with risk factors including traumatic inflammation, fractures, immobilization, and surgical intervention.57 At the same time, multiply injured patients with visceral spinal and head injury also pose a high risk for bleeding.57 The risk is highest among patients with spinal trauma, acute spinal cord injury, or traumatic brain injury.58–60 The baseline risk for VTE in patients with major trauma has been estimated to be at least 3.5%, with higher risk (8–10%) in patients with traumatic brain or spinal cord injury48 Patients with intracranial or subarachnoid hemorrhage are at significant risks for the development of VTE.61–64 In one series, the incidence of VTE was 7.2%, with the incidence of a symptomatic DVT detected by ultrasonography being as high as 24% in patients with subarachnoid hemorrhage.61 Patients with acute intracranial hemorrhage with severe neurologic deficit and a high D-dimer are at increased risk for developing DVT.62 In one meta-analysis, the use of heparins or a heparinoid decreased the incidence of DVT and PE, with a nonsignificant reduction in mortality and a nonsignificant increase in hematoma enlargement in patients with hemorrhagic stroke when compared with placebo or mechanical methods.64 Patients with acute stroke (ischemic or hemorrhagic), particularly with limb paralysis, are at high risk for VTE.65–67 Risk factors peculiar to stroke patients include reduced mobility, elderly age, and multiple comorbid disorders such as heart or respiratory failure, previous myocardial infarction or ischemic stroke, as well as the severity of the stroke as indicated by the National Institutes of Health (NIH) Stroke Scale score.65–67 In all of these disorders, the risk of intracranial or intraspinal spinal bleeding must be weighed against the risk of fatal PE. Modalities shown to be useful in the prevention of VTE in neurosurgical patients include low molecular weight heparin (LMWH), intermittent compression devices, and, in some cases, the use of an inferior vena caval filter. Pregnancy poses a risk for VTE particularly in patients with thrombophilia.35,68–70 The highest-risk thrombophilias include antithrombin deficiency, protein C or S deficiency, homozygosity for the factor V Leiden mutation or antiphospholipid antibody (APLA), and combined defects.70 Other risk factors include prior VTE, multiple birth pregnancies, older age, and cesarean section. VTE can occur in any of the three trimesters, but it is highest in the postpartum period, particularly following cesarean section.39 Venous compression of the left external iliac vein by the iliac artery adds a further risk for thrombosis. In the more extreme form, called the May-Thurner syndrome, thrombosis is a known complication at anytime, but the risk is increased in pregnancy.71,72 Prevention or treatment of VTE in pregnancy requires the use of subcutaneous LMWH throughout pregnancy because of the teratogenic potential of warfarin. Warfarin can be safely used in the postpartum period.35,73,74 The clinical diagnosis of DVT is quite nonspecific because the same symptoms and signs can be caused by other disorders.6,75,76 The usual clinical features of DVT include unilateral calf or leg pain, tenderness and swelling, and occasionally discoloration with venous distention of superficial veins and, in more extreme cases, discoloration including cyanosis.6 The clinical features may not predict the extent of thrombosis, in that some patients with more florid leg signs and symptoms have minimal disease on objective testing, whereas other patients who have extensive venous thrombosis may show few or no clinical signs whatsoever.6 For that reason, objective testing is required on all patients to confirm the diagnosis. Based on prospective studies, pretest probability (PTP) formulas have been developed for the diagnosis of DVT. The most commonly used is the Wells rule (Fig. 10.1).13,77,78 Patients with a score of 2 or less can be categorized as “DVT unlikely,” whereas those with a score of greater than 2 are “DVT likely.” Based on the original studies and systematic reviews, the prospective diagnosis of DVT can be shown to be of low, moderate, or high probability, or “DVT unlikely” or “DVT likely” based on the results of ultrasound testing.78 The results of PTP along with the measurement of the D-dimer can be integrated into diagnostic strategies for the diagnostic management of DVT.13,77,78 The clinical signs and symptoms of PE are also highly nonspecific, and, as with DVT, the diagnosis must be confirmed by objective testing.6,18,79,80 Common symptoms include transient dyspnea and tachypnea, chest tightness or pleuritic pain, and cough (rarely with hemoptysis). In patients with submassive PE, dyspnea and tachypnea may become severe, with right-sided heart failure and cardiovascular collapse with syncope, hypotension, and coma, and in such cases the outcome is frequently fatal.18,19,81 In fact, patients who die of massive PE usually do so within the first 30 to 60 minutes after the onset, when treatment might not have been initiated. For that reason prophylaxis of PE is of upmost importance.18 Fig. 10.1 Diagnostic management of suspected deep vein thrombosis (DVT). U/S, ultrasound. (Adapted from Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013;11:412–422. Reprinted with permission.) As with DVT, there are predictive rules for the diagnosis of PE, which can be confirmed by either CT scanning or ventilation/perfusion (VQ) scanning.82–85 The two clinical decision rules in widespread use are the Wells score82,83 and the Geneva score (Fig. 10.2).84,85 Based on PTP, patients with a score of 4 or less can be categorized as “PE unlikely,” whereas those with a score of more than 4 are “PE likely.”82–85 Prospective management studies have confirmed that patient risk for PE can be accurately divided into low, moderate, or high, or as “PE likely” or “PE unlikely.” The assessment of PTP combined with the D-dimer assessment can be used to streamline the diagnostic approach to the patient with PE. The D-dimer assay is the most useful test in excluding the diagnosis of VTE.86–89 A quantitative D-dimer assay with a rapid turnaround time has been most useful using either an enzyme-linked immunosorbent assay (ELISA) or latex agglutination. The D-dimer test cannot be used to support the diagnosis of VTE because numerous other abnormalities produce positive D-dimer, such as cancer, infection, pregnancy, trauma, surgery, sepsis, and advancing age.87–89 For that reason the D-dimer assay is most useful in streamlining the diagnosis of VTE in outpatients, as most in-hospital patients have a positive D-dimer. The D-dimer assay is used in conjunction with PTP assessment.87–89 Patients with a low PTP and a negative D-dimer may not require further investigations. Fig. 10.2 Diagnostic management of Suspected pulmonary embolism (PE). CTA, computed tomography angiography; U/S, ultrasound. (Adapted from Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013;11:412–422. Reprinted with permission.) Measurement of troponin and brain natriuretic peptide (BNP) provides evidence of myocardial dysfunction associated with PE, and can be useful in determining whether or not patients with PE require thrombolysis or mechanical relief of the vascular obstruction.90 The diagnostic test most commonly used for the diagnosis or exclusion of DVT is ultrasonography32,91–94; less commonly used nowadays is venography.98 Both of these tests have been validated by prospective management studies, which have demonstrated the safety of withholding anticoagulant therapy in patients with negative test results.91–93,95 Ultrasonography is noninvasive and can easily be repeated serially if necessary. It is readily available and can be done at the bedside. Noncompressibility of a vein segment is the most important component of the study, but color flow and venous augmentation can be added. Ultrasound has a high sensitivity and specificity for symptomatic proximal DVT but less for distal DVT (65%), so that repeat examinations are needed to detect propagation into the proximal veins if anticoagulation treatment is not started. Ultrasound has limited value in diagnosing pelvic DVT.32,78 The diagnosis of recurrent DVT is problematic unless a new segment of vein is involved or there is extension of a previous thrombus.39,94–98 Also, ultrasonography may remain abnormal in 30% of patients even at 1 year after an initial event. Measurement of D-dimer may be of value if it is negative, but this requires further study.96,97 Ascending venography may be useful in diagnosing recurrent DVT or in patients in whom repeat ultrasonography is inconclusive or repeat testing is impractical.98 Patients with a low PTP and a negative D-dimer may require no further testing, whereas those with a positive D-dimer require objective tests with ultrasonography. Patients with a high PTP do not undergo D-dimer testing and proceed directly to ultrasonography. If ultrasonography is negative, the patient may require repeat testing or in some cases venography (Fig. 10.1).13,32,77,78,97 Computed tomography venography94 and venography by magnetic resonance imaging (MRI)99 have been useful in demonstrating proximal DVT in conjunction with the diagnosis of PE. MRI of the pelvic veins can be useful, for example, in pregnancy or in patients with isolated iliac vein thrombosis. Computed tomography angiography (CTA) is the diagnostic test of choice for the diagnosis of PE.6,100,101 It is widely available, and has been shown to have a sensitivity of 82 to 100% and specificity of 89 to 98%.100 The sensitivity can be further improved by simultaneously performing CT venography of the lower extremities.100 The technology for CTA has rapidly evolved from single detectors to multiple detectors (64 to 256 rows). Multiple-detector CTA has a higher sensitivity for PE by enabling better visualization of peripheral vessels when compared with single-detector CTA, leading to a higher rate of detection of subsegmental PE.11 As mentioned earlier, the management of these small PE remains controversial and in some cases anticoagulants can be safely withheld.11 Computed tomography angiography has been integrated into the diagnostic strategy for the diagnosis of suspected PE (Fig. 10.2). Some patients with a low PTP and a negative D-dimer may need no further testing, whereas those who have a positive D-dimer require objective testing with CTA. Patients with a high PTP go directly to CTA testing. If the CTA is nondiagnostic, further testing is required with ultrasonography of the legs or in some cases of pulmonary angiography.32,102 Ventilation/perfusion (VQ) lung scanning compares ventilation and perfusion as part of the evaluation for PE.103–106 Perfusion is assessed by injection of isotopically labeled microaggregates of human albumin, and ventilation is assessed by inhaling radioactive aerosol. A mismatch of ventilation and perfusion is considered a high probability for PE. A negative VQ scan is adequate to eliminate the diagnosis of PE. However, more than 70% of patients have a nondiagnostic VQ scan and require further testing, usually by ultrasonography of the legs or performance of CTA. An integrated approach to the diagnosis of suspected PE using VQ scanning is also established.32,102,106 Patients with a low PTP and a negative D-dimer may require no further testing, whereas those with a positive D-dimer have testing with a VQ scan. Those with a high PTP go directly to VQ scanning. If it is nondiagnostic, they will require ultrasonography of the legs, CTA, or, in some cases, pulmonary angiography. VQ scanning is still useful for patients who have anaphylaxis to intravenous contrast media or significant renal impairment or in young women of child-bearing age to eliminate the high radiation exposure to the breasts provided by CTA.32 Another diagnostic test for evaluation of PE is MRI angiography.107 In the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED-III) study, MRI was technically inadequate in 25% of patients despite the fact that all centers were experienced with this technique. Technically adequate MRI had the sensitivity of 78% and the specificity of 99%. With the addition of MRI venography, sensitivity improved to 92% with specificity of 96%, but 52% of patients had technically inadequate results.107 It was hoped that magnetic resonance angiography (MRA), which has no ionizing radiation exposure, would be a useful diagnostic test in pregnant patients.108,109 Although MRI appears to be safe during pregnancy, there have been conflicting data about the safety of gadolinium and fetal development. Also, gadolinium in the presence of renal insufficiency can lead to nephrogenic systemic fibrosis.110 This can cause a progressive thickening and hardening of the skin and other body tissues, including the gastrointestinal tract and the peripheral joints. Therefore, the hope that MRI would be useful in the diagnosis of PE in patients with renal insufficiency where contrast enhanced CTA is contraindicated has not been realized. Pulmonary angiography using selective catheterization of pulmonary arteries is relatively safe in experienced hands and can be used when other approaches are inconclusive or when definitive knowledge about the presence or absence of PE is required urgently, as in patients in the intensive care unit.80 Although echocardiography is not sensitive for the diagnosis of PE, it can be useful in evaluating the evidence of right ventricular dysfunction in the setting of PE.18 Findings on transthoracic echocardiography that suggest right ventricular dysfunction include right ventricular dilatation, hypokinesis, paradoxical intraventricular septal wall motion, tricuspid regurgitation, and pulmonary hypertension. Elevated troponin or BNP results provide further evidence of significant cardiac damage in the presence of PE and can be helpful in assessing patients who may require thrombolysis.90 The objectives of treatment of the patients with VTE are to prevent death from PE, to prevent recurrent VTE, and to prevent the postthrombotic syndrome. Initial anticoagulant treatment of VTE consists of either LMWH or unfractionated heparin given intravenously or by subcutaneous injection for a period of 4 to 5 days in conjunction with an oral anticoagulant that is normally a vitamin K antagonist (VKA), such is warfarin or acenocoumarol, started in conjunction with the heparins.111,112 These are continued until the international normalized ratio (INR) is therapeutic on two consecutive days.111,112 The new oral anticoagulants offer convenient options for both the initial and long-term treatment of VTE.111,112 The LMWHs are derivatives of commercial heparin, with a mean molecular weight of 4 to 5 kd. The LMWHs differ from regular-weight heparin in numerous ways.113,114 Of particular importance are the following: they have increased bioavailability (i.e., > 90% after subcutaneous injection), they have a prolonged half-life, and they have a predictable clearance enabling a once or twice daily injection. Furthermore, there is a predictable antithrombotic response based on body weight, so that treatment can be provided without laboratory monitoring.113,114 The LMWHs that are given by subcutaneous injection have been compared with continuous intravenous unfractionated heparin (UFH) in numerous clinical trials for the treatment of proximal DVT and PE, with long-term follow-up to assess outcome measures. Systematic reviews of these studies indicate that LMWH is as effective as UFH with a reduction in major bleeding and mortality.115 Studies in which LMWH has been used on an outpatient basis have also shown these agents to be effective and safe when compared with intravenous UFH given on an in-patient basis.116–118 LMWH has been shown to be cost-effective for the treatment of VTE when compared with intravenous UFH. LMWH is recommended as the anticoagulant for the initial treatment of VTE by the ACCP.111 Long-term treatment of acute VTE with LMWH has been evaluated in several randomized clinical trials in comparison with oral anticoagulant treatment.119–121 In the two studies in patients with cancer and thrombosis, long-term LMWH for 3 or 6 months was more effective than warfarin in the prevention of recurrent VTE,119,120 and in one study there were fewer bleeding complications.119 In clinical practice guidelines, LMWH is suggested over warfarin for the treatment of thrombosis associated with cancer for the first 3 to 6 months of long-term treatment.111 The anticoagulant activity of UFH depends on a unique pentasaccharide that binds to antithrombin markedly enhancing the inhibition of thrombin and activated factor X (Xa).122,123 Only about one third of the heparin molecules contain the unique pentasaccharide sequence that potentiates the activity of endogenous antithrombin. Heparin also catalyzes the inactivation of thrombin by heparin cofactor 2, which acts independently of antithrombin. The anticoagulant response to UFH varies widely among patients so that it is necessary to monitor the anticoagulant effect either by use of the activated partial thromboplastin time (aPTT) or by heparin levels, and to titrate the UFH dose accordingly. The use of a prescriptive approach such as the weight-based nomogram achieves adequate anticoagulation within the first 24 hours of treatment and results in a reduced rate of recurrent VTE.124–127 The simultaneous use of intravenous or subcutaneous UFH or subcutaneous LMWH with VKA therapy has become standard practice for the initial management of most patients with VTE.122,126,127 Exceptions include patients who are unstable and may require immediate medical or surgical intervention, such as thrombolysis or insertion of a vena caval filter; patients who are at high risk of serious bleeding; and patients who have severe renal insufficiency. Adjusted-dose UFH by subcutaneous injection has been used for the initial treatment of VTE with an efficacy and safety similar to fixed-dose LMWH but is more cumbersome and requires monitoring with the aPTT.128 The LMWH or UFH is continued until the INR is at the therapeutic level for two consecutive days. The main adverse effects of the heparins are bleeding, thrombocytopenia, and osteoporosis. Patients at particular risk of bleeding include are those who have had recent trauma or surgery, and those who have other clinical disorders that predispose them to bleeding, such as peptic ulcer, cancer, liver disease, or hemostatic defects.117 When patients experience bleeding on heparin therapy, their management depends on the location and severity of bleeding, the level of the aPTT, and their risk of recurrent VTE. The heparin infusion should be discontinued temporarily or permanently, and in urgent situations protamine sulfate may be administered. Those patients at risk of recurrent VTE may be candidates for insertion of a temporary of permanent vena caval filter.114 Heparin-induced thrombocytopenia (HIT) is a well-recognized complication of UFH therapy; it is much less common with the use of LMWH. It usually occurs in the first 4 to 10 days after commencing therapy.129 About 1 to 2% of patients receiving UFH will experience a fall in platelet count to less than the normal range or a 50% drop in the platelet count within the normal range. In most of these patients, the thrombocytopenia is mild to moderate and of no clinical significance. It is considered to be a direct effect of the heparin.129–131 However, approximately 0.1 to 0.2% of patients receiving UFH by any route develop an immune thrombocytopenia mediated by an immunoglobulin G (IgG) antibody to a complex of PF4 and heparin. The development of HIT may be accompanied by arterial or venous thrombosis, which can lead to serious consequences including amputation or death. It has been shown that the incidence and severity of HIT varies among different patient populations, being more prevalent in patients having cardiac or orthopedic procedures than in medical patient.131 To aid in the clinical diagnosis of HIT, a formula that assesses the 4 T’s is used,132–134 the 4 T’s being treatment with heparin, timing of the initial dose, the degree of thrombocytopenia, and other factors that can be seen in patients with HIT. When the clinical diagnosis of HIT is made, heparin in all forms must be stopped immediately. In most centers a screening test, which is an ELISA for the heparin-platelet factor 4 complex, can be measured. Because there are frequent false-positive test results with the ELISA test, a confirmatory test should be performed if available, and the most accurate of these tests is the serotonin release assay. Both of these tests require lengthy times before the results are available, so that management must be instituted on clinical grounds. For patients requiring ongoing anticoagulation, there are several choices, including the use of a specific antithrombin agent, argatroban,135 or the direct thrombin inhibitor lepirudin.136 More recently, fondaparinux has been shown to be useful for the treatment of HIT.137 Osteoporosis can occur in patients on UFH, particularly those taking it for 6 months or more and those who receive therapeutic doses. Bone demineralization can lead to vertebral fractures or fractures of long bones, and this defect may not be reversible.122 Osteoporosis has not been seen with the long-term use of LMWH.138 Fondaparinux is a synthetic inhibitor of factor Xa that markedly increases the activity of antithrombin and therefore differs from LMWH.114 It is rapidly absorbed following subcutaneous injection and has an elimination half-life of 17 to 21 hours in healthy subjects and is prolonged in patients over the age of 75. About 77% of the drug is excreted unchanged in the urine, and drug levels increase with decreasing renal impairment.114 Therefore, fondaparinux is contraindicated in patients with severe renal insufficiency (creatinine clearance [CrCl] < 30 mL/min) and should be used with caution in patients with mild to moderate renal failure. Fondaparinux has been approved as a substitute for unfractionated heparin or LMWH for the initial treatment of VTE.139,140 However, fondaparinux has not replaced LMWH for the initial treatment of VTE in most countries mainly because of the prolonged half-life, the concern about using it in patients with renal insufficiency, and the fact that the anticoagulant effect cannot be blocked. The anticoagulant effect of warfarin is mediated by the inhibition of vitamin K–dependent gamma-carboxylation of coagulation factors II, VII, IX, and X, and warfarin treatment results in the synthesis of immunologically detectable but biologically inactive forms of these coagulation proteins.112,141,142 Warfarin also inhibits the biological activity of proteins C and S, which are responsible for the inhibition of the coagulation process by activated protein C. Because the activity of proteins C and S are inhibited before there is adequate anticoagulant effect on the other clotting factors, it is mandatory to inhibit the coagulation system with an agent such as LMWH or fondaparinux for a period of time until there is adequate suppression of all the vitamin K–dependent factors.112 As the dose-response relationship varies widely among patients, the dose must be monitored with the use of a laboratory test143—the INR. The range of the INR for the treatment of VTE is 2 to 3. There is a relatively good relationship between recurrent thrombosis when the INR is in the subtherapeutic range and spontaneous bleeding when the INR becomes significantly elevated.112 Numerous drugs may interfere with warfarin therapy, but a critical appraisal of the literature indicates that such interactions tend to be poorly supported by evidence in most cases.144 There are several genetic polymorphisms that may make patients more or less sensitive to warfarin such as CVP2C9, CVP4F2, or VKORC1.145 More frequent determinations of INR are required with the initial use of warfarin, but once the anticoagulant effect is stable INR may be measured in 2 to 4 weeks, and many patients do not require many dose adjustments. There are warfarin nomograms and computer software programs that assist caregivers in the control of warfarin therapy.115 Also, the use of self-testing with portable INR monitors can simplify management of warfarin, and in many cases patients can self-manage their warfarin therapy. Anticoagulant management clinics have also improved the quality of warfarin treatment.112 The appropriate duration of anticoagulant treatment for VTE has been evaluated in numerous trials.111,146–150 These trials have demonstrated that warfarin therapy is highly effective in preventing recurrent VTE, but there is an increase in the risk of bleeding.146–150 The current approach to the treatment of VTE is to continue oral anticoagulant therapy for a full 3 months to suppress the coagulation system. This is referred to as initial treatment, and anything beyond this is referred to as extended treatment.111,151 Patients who require anticoagulation therapy beyond the extended period are in long-term treatment.111,151 Patients who have VTE secondary to a precipitating event such as surgery or trauma have a lower incidence of recurrent VTE, and the usual treatment period of anticoagulation is only 3 months.111 Patients with the first episode of unprovoked or idiopathic VTE should be treated for at least 3 months. For those patients at high risk of bleeding, 3 months may be the duration of anticoagulant therapy. However, in most patients, the treatment period is extended for 6 to 12 months.111 The decision regarding extended and long-term treatment must be individualized, taking into consideration the estimated risk of recurrent VTE and the risk of bleeding as well as patient compliance and preference. In all cases the anticoagulant management program must be reviewed periodically with respect to risk benefit assessment.111 Patients who have a second episode of unprovoked VTE usually require indefinite anticoagulation unless the bleeding risk is high, but in all cases the treatment program should be reviewed periodically.111 Bleeding is the major side effect of oral anticoagulant therapy.111,152–154 The most important factor leading to the bleeding risk is the intensity of the INR, with clinically important bleeding being higher when the targeted INR increases above 4.5. Other factors include a history of bleeding, previous stroke or myocardial infarction, hypertension, renal failure, and diabetes.151 There is a strong negative relationship between the percentage of time that patients are within the targeted INR range and both bleeding and a recurrent thrombosis.154 Oral anticoagulant therapy in the elderly presents further tendencies to increased bleeding, as do the presence of cancer, intestinal polyps, and renal failure.155 Bleeding in patients on VKA therapy with an elevated INR must be evaluated for the degree of elevation of the INR and the clinical circumstances.112,156 The options available include temporary interruption of the warfarin treatment, or the administration of vitamin K or of blood products such as fresh frozen plasma or prothrombin complex concentrates. If the INR is mildly increased and the patient is not bleeding, no specific therapy is necessary other than reducing the warfarin dose. With a more marked increase of the INR in patients who are not bleeding, treatment with small doses of vitamin K (e.g., 1 mg per day) given either orally or subcutaneously should be considered.112 With more marked increase in the INR, particularly in patients who are actively bleeding, the coagulation defect should be corrected. Vitamin K can be given intravenously or subcutaneously. If the bleeding defect requires further correction, fresh frozen plasma may be given, or, if anticoagulation should be more rapidly reversed, prothrombin complex concentrates can be given intravenously.112 If prothrombin complex concentrates are not readily available, recombinant activated factor VII has been used to control life-threatening bleeding.157–159 Patients on long-term anticoagulation frequently require temporary interruption of treatment for surgery or other invasive procedures.112 The risk of recurrent VTE when anticoagulants have been discontinued must be weighed against the risk of bleeding if either UFH or LMWH is administered before or after the surgical procedure or if oral anticoagulant therapy is continued at therapeutic levels.112,160–163 The approach chosen will depend on the risk benefit assessment of each individual patient. Options include continuing warfarin at therapeutic doses for procedures such as most dental extractions, skin biopsies, or ophthalmologic procedures; lowering the warfarin dose to maintain an INR in the lower or subtherapeutic range during the surgical procedure; and discontinuing warfarin for 3 to 5 days before the procedure to allow the INR to return to normal, with or without bridging with LMWH at prophylactic or therapeutic doses before the procedure and then after the procedure until warfarin therapy is therapeutic.160 Recently, there has been much interest in the development of new antithrombotic agents that may be able to replace warfarin.112,114,164–168 The most advanced agents are specific inhibitors of activated factor X (Xa) or thrombin (IIa).114,164–168 These agents have the advantage that they can be given by the oral route once or twice daily, they require no laboratory monitoring, and, in most cases, the same dose is taken by all patients. The agents available at present are either factor Xa inhibitors: rivaroxaban (Xarelto; Bayer, Johnson & Johnson),166 apixaban (Eliquis; BMS/Pfizer),167 and the antithrombin inhibitor dabigatran etexilate (Pradax/Pradaxa; Boehringer Ingelheim International).168 The clinical pharmacology of rivaroxaban, apixaban, and dabigatran is shown in Table 10.1. Rivaroxaban is a direct inhibitor of activated factor X.114,164,166 It has about 80% absorbability, and peak blood levels appear within 2 to 3 hours.166,169 The terminal half-life is 7 to 11 hours. Approximately 33% of the drug is eliminated by the kidney. Following an oral dose of rivaroxaban, there is a direct relationship between pharmacodynamic effects and the degree of renal impairment.166 In patients with severe renal insufficiency (CrCl < 30 mL/min), the area under the curve (AUC), indicating increased rivaroxaban concentration, was increased by 1.2 to 2.2.166 For this reason, rivaroxaban must be used with caution in patients with moderate renal impairment (CrCl 30–49 mL/min), and it is contraindicated with a CrCl < 30 mL/min.166 The pharmacokinetic/pharmacodynamic (PK/PD) profile of rivaroxaban is dose dependent and predictable, with no change with age, sex, or body weight.169 In clinical trials there has been no evidence of liver or other toxicities and no increase in vascular events such as myocardial infarction, stroke, or peripheral thrombosis.169 Apixaban is also a direct inhibitor of factor Xa, with an oral availability of about 50%.112,114,167,170–172 Peak blood levels are seen within 3 to 4 hours, and the terminal half-life is 8 to 12 hours. Approximately 27% of the drug is eliminated by the kidney. Apixaban dose adjustment is not required for patients with mild to moderate renal impairment, but it is contraindicated in a patient with a CrCl < 30 mL/min.167 No dose adjustment is required for age or weight, and in clinical trials there is no evidence of hepatic or other toxicities and no increase in the incidence of vascular events.167 Dabigatran etexilate is a prodrug that is rapidly converted to active dabigatran in the liver following absorption.112,114,164,165,168,173–177 Bioavailability is about 6.5%, and peak blood levels are reached within about 2 hours. The terminal half-life is about 14 to 17 hours, with most of the drug (80%) eliminated through the kidney unchanged. There is a direct correlation between the systemic exposure to dabigatran and the degree of renal insufficiency.168 Moderate renal impairment (CrCl 30–50 mL/min) increased the AUC twofold, whereas severe renal impairment (CrCl < 30 mL/min) increased it sixfold.168 Dabigatran has predictable PK/PD profiles without any influence of weight or sex and no food interactions.112,114,168,174–176 Dabigatran is not metabolized through the cytochrome P-450 3A4 (CYP3A4) pathway, but inhibitors or inducers of P-glycoprotein can affect drug levels. Dabigatran should be used with caution in patients with CrCl between 30 and 49 mL/min, and it is contraindicated in those with a CrCl < 30 mL/min.177 It is recommended that CrCl measurements be made before starting dabigatran and periodically during treatment, particularly in elderly patients with borderline renal function. In clinical trials and in practice, dabigatran treatment has been complicated by dyspepsia, which has led to the discontinuation of treatment in several patients.168 There is also a higher incidence of lower gastrointestinal bleeding, which may be related to the high concentration of active dabigatran excreted in the stool. A higher rate of myocardial infarction has been observed in two clinical trials of dabigatran when compared with warfarin and enoxaparin treatment.178–181 A meta-analysis of seven clinical trials of dabigatran supported the findings of the individual trials.180 Because dabigatran has been widely used, there have been reports of excessive bleeding in the medical literature and in reports to the Food and Drug Administration (FDA). There is concern that the drug effect cannot be blocked; for example, patients who are elderly with borderline renal function that may rapidly deteriorate are those most at risk for excessive bleeding on this drug.112,165 Although routine monitoring of the drug effect for the new oral anticoagulants (NOACs) is not required, there are times when it is important to assess the drug effect for a risk of bleeding or thrombosis. Examples of such occasions are the need for emergency or urgent intervention for surgery, serious bleeding or a thrombotic event, worsening renal or hepatic function, suspected overdosing, or drug interactions.165 An elevated aPTT or prothrombin time (PT) can provide qualitative evidence of the presence of dabigatran or the factor Xa inhibitors, respectively, but each lacks sensitivity for a quantitative evaluation.165,182–184 A calibrated prothrombin time using a neoplastin or neoplastin plus reagent can provide a good correlation with plasma concentration of rivaroxaban,165,183 but there are no data on apixaban plasma levels with this test. For both factor Xa inhibitors, the most reliable measure of plasma levels is a chromogenic Xa level, which is available in most larger centers.165,185 However, at this time there are no studies correlating Xa levels taken at peak or trough times with either bleeding or thrombotic events.165 The best test available to measure the drug levels of dabigatran is the dilute thrombin time (DTT), which is now commercially available (the Hemoclot test).165 A normal DTT indicates no detectable dabigatran, and an elevated level (e.g., > 200 ng/kg) indicates an increased risk of bleeding. At this time there are no data to indicate a safe plasma drug level for surgical intervention.165 When interpreting the results of these laboratory tests, it is essential to know the timing of the last dose, the dosage used, the level of renal and hepatic function, and the risk of bleeding. When possible, the most effective approach to an intervention is to delay the procedure until the drug effect is gone (e.g., 12 to 24 hours depending on the level of renal function and the required level of hemostasis). In urgent situations, general measures include fluid replacement, which may consist of packed red blood cells, platelets, fresh frozen plasma, colloids, surgical hemostasis, or compression if possible.165 In the emergency situation prothrombin complex concentrates (PCCs) or activated factor VII may be given.165,186–189 There is no evidence that PCCs block the anticoagulant effect of dabigatran in humans, but in healthy volunteers the anticoagulant effect of rivaroxaban was effectively blocked by PCC.186 However, there are no data on the effect of PCCs in patients with serious bleeding. There is early evidence that a catalytically inactive factor Xa that can bind to the direct factor Xa inhibitors can reverse the anticoagulant activities of rivaroxaban or apixaban.191 There is little to support the use of activated factor VII (e.g., NovoSeven in either the Xa or thrombin inhibitors).165 In patients on hemodialysis who received dabigatran prior to dialysis, the drug level was lowered by 60% after 2 hours’ treatment, and there are case reports supporting the role of hemodialysis in this setting.165,168 There is preliminary evidence that an antibody against dabigatran and a hapten can effectively block the anticoagulant activity of dabigatran.168 When the factor Xa or IIa inhibitors must be discontinued for procedural reasons, such as surgery, biopsies, or insertion of a pacemaker, it is recommended that the drug should be stopped in time to allow four to five drug half-lives before the procedure, particularly if minimal drug effect is required.165–167 For dabigatran, this translates into 3 days before surgery if the CrCl > 50 mL/min and 4 to 5 days for a CrCl of 30 to 49 mL/min.167 For rivaroxaban and apixaban in patients with mild to moderate renal impairment (CrCl > 50 mL/min), the last dose should be given 3 days before surgery. In patients in whom a mild to moderate anticoagulant effect of the drug is acceptable, the drug may be continued for an extra day before discontinuing.165,166 When the drugs are to be resumed, it is recommended that they be delayed for at least 48 hours after major surgery and 24 hours after minor surgery. In all cases, the determination of risk of bleeding must be weighed against the risk of thrombosis.165–168 All three agents have been compared with standard VKA therapy for the prevention of stroke and atrial fibrillation178,189,190 and for the initial and extended treatment of VTE.179,191–195 Dabigatran, rivaroxaban, and apixaban are available for use in several countries for the prevention of stroke in patients with atrial fibrillation. Dabigatran and rivaroxaban have been approved or are under review for treatment of DVT and PE, whereas clinical trials in the treatment of VTE with apixaban are just being reported. In the Einstein DVT and PE studies, rivaroxaban 15 mg twice daily was administered for 3 weeks followed by 20 mg once daily and compared with standard VTE therapy with a targeted INR of 2 to 3.179,193,194 Although there was no initial use of LMWH bridging in the rivaroxaban arm, patients were eligible for the study if they had received therapeutic doses of LMWH, fondaparinux, or UFH for less than 48 hours or if they had received no more than a single dose of VKA therapy before randomization. In the Einstein DVT study, rivaroxaban was shown to be noninferior to VKA therapy in the prevention of recurrent VTE with similar bleeding rates.193,194 In patients who had completed 6 to 12 months of treatment with rivaroxaban, this agent was compared with placebo in the prevention of recurrent VTE for an additional 6 to 12 months. Rivaroxaban significantly reduced the incidence of VTE in these patients, but there was an increase (nonsignificant) in major bleeding. In the Einstein PE study, rivaroxaban was shown to be noninferior to warfarin in the prevention of recurrent VTE.194 The rates of the principal safety end point of a composite of major and nonmajor clinically relevant bleeding were similar, but the rates of major bleeding alone were significantly reduced by rivaroxaban.193,194 Three studies have been reported on the use of dabigatran etexilate in the treatment of VTE.182,194,195 In the RE-COVER trial, dabigatran 150 mg twice daily was compared with standard warfarin therapy with a targeted INR of 2 to 3.191 Patients presenting with VTE were given parenteral anticoagulation therapy with UFH or LMWH for 8–10 days before either the dabigatran etexilate or warfarin were started. Treatment continued for 6 months without follow-up for 30 days following that period. Dabigatran was shown to be noninferior to the standard VKA therapy in the prevention of recurrent VTE or VTE-related death, and the incidence of major bleeding was comparable. The incidence of the composite of major and nonmajor clinically relevant bleeding, however, was significantly less with dabigatran.191 In two studies on the extended treatment of VTE, dabigatran when compared with warfarin had decreased recurrent VTE and resulted in less major and clinically relevant nonmajor bleeding.179,192 However, there was a higher incidence of acute coronary syndromes, as had been recorded in previous trials comparing dabigatran with warfarin.179 When compared with placebo, the incidence of recurrent VTE was lower, but the incidence of clinically relevant nonmajor bleeding was higher.179,192 Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months was compared with conventional treatment (LMWH followed by warfarin) for 6 months. The incidence of VTE and VTE-related death was noninferior with apixaban therapy, and there was significantly less major bleeding and a composite of major and clinically relevant nonmajor bleeding.195 When these new oral antithrombotic agents become available for the treatment of VTE, they will provide a convenient alternative to VKA therapy, particularly in those in whom anticoagulant management is difficult or impossible to achieve. Until there is more experience with the new oral antithrombotic agents, they would not be recommended for the treatment of VTE associated with neurosurgical conditions such as brain tumors, intracranial bleeding, traumatic brain injuries, and spinal cord injuries, but with more clinical experience their use will undoubtedly become more widespread. Recent clinical trials on the use of catheter-directed thrombolysis (CDT), with or without mechanical thrombus fragmentation, in patients who have extensive iliofemoral DVT, have shown that CDT improves vein patency and venous valve function in the initial and follow-up period in a number of these patients.196–199 Furthermore, there is evidence that CDT can reduce the incidence of the PTS111,196–199 and can improve the quality of life in these patients.112,198 Operative thrombectomy has been shown to improve vein patency with less leg swelling and fewer venous ulcers when compared with anticoagulation alone.196,200 Patients with iliofemoral DVT who are candidates for these more invasive approaches to therapy include those with DVT of less than 14 days’ duration, particularly if venous gangrene is imminent, who have good functional status, and who are at low risk of bleeding. Following the initial treatment, patients are treated with anticoagulations in the usual way.112,200 CDT may cause less major bleeding and, in particular, a lower incidence of intracranial hemorrhage than systemic thrombolysis. At this time the ACCP recommendation is for anticoagulation therapy over CDT, systemic thrombolysis, or venous thrombectomy.111 Although individual studies and meta-analyses of various studies comparing systemic thrombolysis with heparinization alone in patients with PE have demonstrated improvement in pulmonary vascular resistance with thrombolysis but no mortality advantage,201 there are many contraindications to systemic thrombolysis, including intracranial bleeding or recent stroke, recent surgery or trauma, pregnancy, cancer, recent needle biopsy of internal organs, and hemostatic defects.201,202 The use of systemic or local thrombolysis for the treatment of submassive PE should only be undertaken after careful consideration of the risk/benefit of the procedure to the patient.201 Mechanical dissolution of the embolus may be carried out in conjunction with thrombolysis.202 Insertion of a removable or permanent inferior vena cava (IFC) filter is indicated for patients who have an acute VTE and have an absolute contraindication to anticoagulation, such as major bleeding or objectively documented recurrent VTE during adequate anticoagulation therapy.111,203–207 Although IVC filters have not been shown to decrease mortality, they can effectively prevent important pulmonary embolism. In follow-up studies, although there is a decreased incidence of PE,111,203 there is an increased incidence of recurrent DVT and IVC filter thrombosis.204,205 There is also evidence that IVC filter use may increase the incidence of the PTS.208 Long-term anticoagulation therapy should be started as soon as safely possible after placement of a vena cava filter and continued for the duration of treatment as for patients without an IVC filter.111 There have been numerous clinical trials and meta-analyses of the prevention of VTE in neurosurgical patients.48,209,210 These include patients with traumatic brain injury, intracranial hemorrhage, craniotomy for brain tumors, traumatic head injuries, and central nervous system lymphomas. For most patients, the use of LMWH, with or without sequential compression devices, has been the recommended approach to thromboprophylaxis.48,209,210 There has been a paucity of clinical trials and observational studies on the treatment of VTE in these patients. In all patients the risk of inducing or promoting intracranial bleeding must be weighed against the risk of VTE and, in particular, death from PE. In patients at high risk of bleeding who have VTE, the ACCP recommends anticoagulant treatment for only 3 months.111 Systemic thrombolysis is contraindicated in neurosurgical patients, and any form of anticoagulation is contraindicated when there is active intracranial bleeding.209 Anticoagulant therapy should be used with caution in patients with recent intracranial surgery, those with preexisting hemostatic defects such as thrombocytopenia, those who are at high risk of falls, and those who have poor compliance with medical therapy.48,209,210 However, patients with an intracranial tumor or brain metastases without evidence of active bleeding and who have VTE are candidates for anticoagulant therapy because of the high incidence of fatal PE.48,209 These patients require careful monitoring to limit the risk of bleeding complications, particularly with over-anticoagulation209,210 There have been a number of reports of the use of anticoagulants or vena caval filters (or both) in patients with primary or secondary brain tumors and the presence of VTE.211–217 Most studies are retrospective, with small numbers of patients; none are randomized, and the patient populations are heterogeneous.210–217 Anticoagulant therapy consisted of UFH in various doses or LMWH for short- or long-term use. In some cases VKA therapy has been added.212,213,215 Reported rates of recurrent VTE and bleeding have been low,49,216 and in particular the more recent reports have recorded low or absent incidence of intracranial hemorrhage.212,213,216 In one study the use of vena caval filters alone resulted in a high incidence of thrombotic complications (VTE and filter thrombosis).214 In a nonrandomized study comparing patients who had IVC filters or anticoagulant therapy, there was no differences in in-hospital or overall mortality between the two treatment groups.217 In a recent retrospective review of the use of LMWH in patients with brain metastases and VTE or superficial thrombosis, there was no evidence of clinical or radiographic findings of intracranial hemorrhage.216 Based on evidence from randomized clinical trials, it has been recommended that patients with VTE and cancer should receive treatment with LMWH for 3 to 6 months.111 Following this initial treatment, the decision must be made as to whether to continue LMWH or switch to oral anticoagulant therapy. At this time the use of the new oral anticoagulants is not recommended. In those patients with ongoing metastatic malignancy including brain metastases, the recommendation is to continue LMWH therapy.111 In patients with proximal DVT in the presence of active bleeding or a high risk of bleeding, a retrievable IVC filter may be inserted until the risk of bleeding subsides, at which time the filter can be removed and anticoagulant therapy may be started.112 Although DVT usually occurs in the deep veins of the lower extremities, thrombosis may occur in a number of unusual locations.7,8,44,218 Upper extremity DVT (UEDVT) may involve the axillary and more proximal veins, and the treatment with LMWH and VKA therapy is recommended rather than no treatment or thrombolytic treatment.44,218 If thrombolysis is administered, patients should undergo anticoagulation in the usual intensity and duration as for those who do not have thrombolysis treatment. When UEDVT is associated with central venous catheters, the recommendation is to leave the catheter in place if it is functional, and to initiate the anticoagulation for a period of at least 3 months.111 The same recommendation for 3 months of anticoagulation applies to patients in whom the catheter is removed or left in situ and for those with an underlying malignancy.111 Isolated iliac vein thrombosis may occur spontaneously or as a result of iliac vein compression in pregnancy or in the May-Thurner syndrome.71,72 Depending on the circumstances, treatment may include thrombolysis with mechanical thrombectomy or, in the case of the May-Thurner syndrome, stenting of the involved of narrowed segment of the iliac vein.71–73 Otherwise, treatment with LMWH and VKA is recommended. Thrombosis may occur in the splanchnic bed involving the portal splenic and mesenteric veins. If these are symptomatic they require anticoagulation therapy for at least 3 months or until the original thrombosis has resolved.111 For thrombosis found incidentally in patients who are not symptomatic, no anticoagulation therapy is recommended.111 For patients with calf vein thrombosis distal to the popliteal vein, the recommendation is to treat with anticoagulant therapy for at least 3 months or to follow-up with repeat ultrasound.4,111 Although superficial vein thrombosis has been considered a relatively benign disorder that has been treated with nonsteroidal anti-inflammatory drugs, compression stockings, and rest, there is evidence that superficial vein thrombosis (SVT) can have more serious consequences, such as the development of PE and proximal DVT, especially when it involves the veins in the thigh.9,219 This is particularly true for SVT of the lower limb of at least 5 cm in length if it is in close proximity to the groin. In such patients, the use of prophylactic doses of fondaparinux or LMWH when compared with placebo significantly decreased extension of the original SVT.219 Patients who have extensive SVT close to the saphenofemoral junction, a history of previous DVT or SVT, active cancer, or recent surgery are candidates for prophylactic anticoagulation treatment for up to 45 days. • The serious consequences of VTE are fatal pulmonary embolism, postthrombotic syndrome, chronic thromboembolic hypertension, and paradoxical cerebral embolism. • Postthrombotic syndrome occurs more commonly in patients with recurrent ipsilateral ileofemoral DVT and in those who have inadequate anticoagulation in the early state of treatment. • Thrombophilias include factor V Leiden, prothrombin mutant, antithrombin deficiency, protein C or S deficiency, and antiphospholipid antibody syndrome. • Common risk factors for VTE in neurosurgery: • Clinical signs and symptoms for VTE are nonspecific and diagnosis must include objective diagnostic tests that have been properly validated, • Objective tests for DVT: • Objective tests for PE: • Echocardiography and biomarkers assess right heart strain and can determine which patients with PE may benefit from early thrombolysis/mechanical dissolution therapy. • Treatment of venous thromboembolism: • Catheter-directed thrombolysis (CDT) with or without mechanical clot dissolution: • D-dimer test to exclude VTE: • Removable or permanent IVC filters are usually indicated in patients with VTE who have absolute contraindications to anticoagulants or who have major bleeding while anticoagulated and in some cases of extension or new thrombus formation while anticoagulated. • Treatment of VTE in neurosurgery patients:
Venous Thromboembolism (DVT and PE): Diagnosis and Treatment
Definition
Incidence
Serious Consequences of Venous Thromboembolism
Fatal Pulmonary Embolism
Postthrombotic Syndrome
Chronic Thromboembolic Pulmonary Hypertension
Etiology and Pathogenesis
Risk Factors for Venous Thromboembolism
Risk Factors for Venous Thromboembolism in Neurosurgery Patients
Brain Tumors
Spinal Surgery
Trauma Patients with Spinal Cord Injury
Intracranial Hemorrhage
Stroke Patients
Venous Thromboembolism and Pregnancy
Diagnosis of Venous Thromboembolism
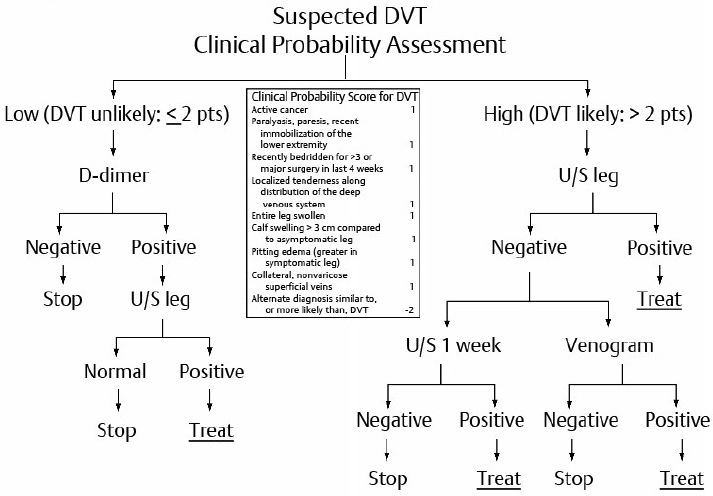
Pretest Probability Assessment of Deep Vein Thrombosis
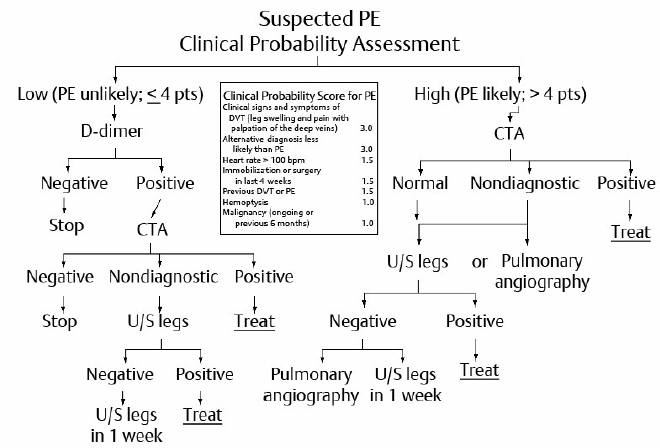
Pretest probability Assessment of Pulmonary Embolism
D-Dimer Assay
Other Laboratory Tests
Objective Testing for Deep Vein Thrombosis
Objective Testing for Pulmonary Embolism
Computed Tomography Angiography
Ventilation/Perfusion Lung Scanning
Magnetic Resonance Imaging
Pulmonary Angiography
Echocardiography and Biomarkers
Treatment of Venous Thromboembolism
Heparin Therapy
Low Molecular Weight Heparin as the Initial Treatment
Long-Term Low Molecular Weight Heparin Treatment
Unfractionated Heparin Treatment
Fondaparinux
Long-Term Treatment with Vitamin K Antagonist—Warfarin
Duration of Anticoagulant Therapy and Recurrent Venous Thromboembolism
Adverse Effects of Oral Anticoagulants
Management of Over-Anticoagulation
Management of Patients on Long-Term Oral Anticoagulant Therapy Who Require Surgical Intervention
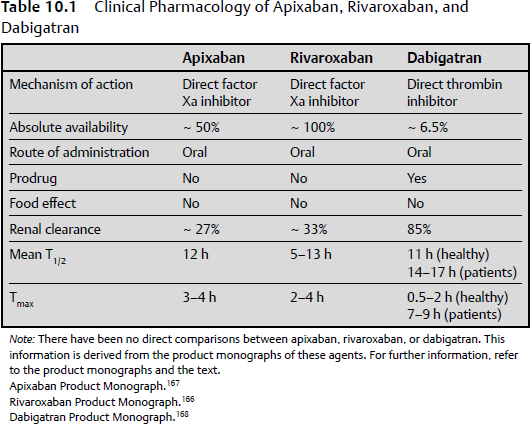
New Oral Anticoagulants
Factor Xa Inhibitors (Rivaroxaban and Apixaban)
Factor II Thrombin Inhibitor (Dabigatran)
Measuring the Anticoagulant Effect of the New Oral Anticoagulants
Management of Emergency Situations Requiring Reversal of the Anticoagulant Effect
Interruption of Treatment with the New Oral Antithrombotic Agents
New Oral Anticoagulants in the Treatment of Venous Thromboembolism
Thrombolysis or Mechanical Fragmentation for the Treatment of Proximal Deep Vein Thrombosis and Pulmonary Embolism
Inferior Vena Cava Filter
Treatment of Venous Thromboembolism in Neurosurgery Patients
Thrombosis in Unusual Locations
 Craniotomy (particularly for brain tumor
Craniotomy (particularly for brain tumor
 Higher grade malignancy
Higher grade malignancy
 Metastatic malignancy
Metastatic malignancy
 Chemotherapy or radiation therapy
Chemotherapy or radiation therapy
 Immobilization
Immobilization
 Multiple trauma
Multiple trauma
 Advanced age
Advanced age
 Compression ultrasound (US) is the most useful test for the diagnosis of DVT with high sensitivity and specificity for symptomatic proximal DVT.
Compression ultrasound (US) is the most useful test for the diagnosis of DVT with high sensitivity and specificity for symptomatic proximal DVT.
 Complete compression US can detect distal DVT is symptomatic patients.
Complete compression US can detect distal DVT is symptomatic patients.
 Ascending contrast venography may be occasionally required for the diagnosis of recurrent DVT or when repeat US is impractical or inconclusive.
Ascending contrast venography may be occasionally required for the diagnosis of recurrent DVT or when repeat US is impractical or inconclusive.
 CT or MRI venography may be helpful in diagnosing DVT proximal to the inguinal ligament (e.g., isolated iliac vein thrombosis, with pregnancy or with vessel anatomical abnormalities/defects such as May-Thurner syndrome).
CT or MRI venography may be helpful in diagnosing DVT proximal to the inguinal ligament (e.g., isolated iliac vein thrombosis, with pregnancy or with vessel anatomical abnormalities/defects such as May-Thurner syndrome).
 Multidetector CT angiography is the diagnostic test of choice, with a high sensitivity and specificity for the diagnosis of symptomatic PE.
Multidetector CT angiography is the diagnostic test of choice, with a high sensitivity and specificity for the diagnosis of symptomatic PE.
 Adding CT venography to CTA increases the sensitivity.
Adding CT venography to CTA increases the sensitivity.
 VQ scanning has a role in the diagnosis of PE in patients with renal insufficiency, those who have allergy to contrast agents, and in younger women because of the concern about radiation exposure to the breasts with CTA.
VQ scanning has a role in the diagnosis of PE in patients with renal insufficiency, those who have allergy to contrast agents, and in younger women because of the concern about radiation exposure to the breasts with CTA.
 MRI angiography has a limited role in the diagnosis of PE in pregnancy but has not become routine.
MRI angiography has a limited role in the diagnosis of PE in pregnancy but has not become routine.
 Pulmonary angiography is occasionally required (e.g., when a positive diagnosis is urgently needed in ICU patients).
Pulmonary angiography is occasionally required (e.g., when a positive diagnosis is urgently needed in ICU patients).
 Standard therapy: initiate LMWH or UFH and warfarin therapy simultaneously. Continue for at least 5 days or until INR is in the therapeutic range for 2 consecutive days.
Standard therapy: initiate LMWH or UFH and warfarin therapy simultaneously. Continue for at least 5 days or until INR is in the therapeutic range for 2 consecutive days.
 Alternative therapy strategy: LMWH in therapeutic doses once or twice daily for at least 3 months (may be followed by warfarin therapy or continued LMWH).
Alternative therapy strategy: LMWH in therapeutic doses once or twice daily for at least 3 months (may be followed by warfarin therapy or continued LMWH).
 LMWH is the recommended treatment of cancer-related VTE and is the preferred approach to VTE treatment in neurosurgery.
LMWH is the recommended treatment of cancer-related VTE and is the preferred approach to VTE treatment in neurosurgery.
 Indicated in proximal DVT with impending vascular insufficiency.
Indicated in proximal DVT with impending vascular insufficiency.
 May be indicated in isolated ileofemoral DVT of recent onset (7–14 days) if there is good functional status and low risk of bleeding.
May be indicated in isolated ileofemoral DVT of recent onset (7–14 days) if there is good functional status and low risk of bleeding.
 May be indicated in patients with submassive PE with none of the usual contraindications.
May be indicated in patients with submassive PE with none of the usual contraindications.
 A quantitative D-dimer test with a rapid turnaround (e.g., ELISA assay) is the most useful.
A quantitative D-dimer test with a rapid turnaround (e.g., ELISA assay) is the most useful.
 A negative D-dimer test result has a very high negative predictive value in excluding VTE.
A negative D-dimer test result has a very high negative predictive value in excluding VTE.
 Most hospitalized patients have a positive D-dimer, rendering it of little use excluding VTE.
Most hospitalized patients have a positive D-dimer, rendering it of little use excluding VTE.
 The combination of a D-dimer and a pretest probability test directs the diagnostic management of VTE.
The combination of a D-dimer and a pretest probability test directs the diagnostic management of VTE.
 First weigh the risk of bleeding (especially intracranial) against the risk of fatal PE.
First weigh the risk of bleeding (especially intracranial) against the risk of fatal PE.
 In most cases, long-term LMWH is the preferred approach as opposed to warfarin treatment.
In most cases, long-term LMWH is the preferred approach as opposed to warfarin treatment.
 Use of systemic or regional thrombolysis is contraindicated.
Use of systemic or regional thrombolysis is contraindicated.
 Use of fondaparinux or the new anticoagulants are strongly discouraged because of the difficulty in blocking the anticoagulant effect (particularly so with fondaparinux and dabigatran).
Use of fondaparinux or the new anticoagulants are strongly discouraged because of the difficulty in blocking the anticoagulant effect (particularly so with fondaparinux and dabigatran).
 Use of an IVC filter ± anticoagulants is an alternative approach.
Use of an IVC filter ± anticoagulants is an alternative approach.
 Future prospective clinical trials are clearly indicated to determine the appropriate management.
Future prospective clinical trials are clearly indicated to determine the appropriate management.
Venous Thromboembolism (DVT and PE): Diagnosis and Treatment
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


 Monitor the warfarin effect with the INR starting on day 3 and periodically throughout treatment for at least 3 months, although extended or long-term treatment may be indicated.
Monitor the warfarin effect with the INR starting on day 3 and periodically throughout treatment for at least 3 months, although extended or long-term treatment may be indicated.




