Fig. 11.1
Anatomy of the vertebral artery [modified from: Gray H, Lewis WH. Anatomy of the human body (20th ed). Philadelphia, PA: Lea & Febiger 1918]
The VA is anatomically and angiographically divided into 4 segments:
V1 (ostial segment), from its origin to its entry into the foramen transversarium of C6;
V2 (foraminal segment), from its entry into the foramen transversarium of C6 to its exit from the foramen transversarium of C2;
V3 (suboccipital segment), from its exit from the foramen transversarium of C2 to its dural entry;
V4 (intradural segment), from its entry into the dura to the vertebrobasilar junction.
V2 is the longest segment, which is surrounded and protected by bone throughout most of its course. Therefore, although relatively less vulnerable to injury than other VA segments, it remains the most difficult to access surgically. Throughout their course, the V2 and V3 segments of the VA are surrounded by a dense vertebral venous plexus and give rise to muscular branches that anastomose with those of other major cervical arteries, including the ascending and deep cervical arteries (branches of the thyrocervical and costocervical trunks of the subclavian artery, respectively), and the ascending pharyngeal and occipital arteries (branches of the external carotid artery (ECA) ). The V3 segment also often gives rise to the posterior meningeal artery, which vascularizes the dura of the foramen magnum and posterior fossa.
Given that a robust collateral circulation usually exists in the vertebrobasilar system, including a contralateral vertebral artery and 2 posterior communicating arteries (PComAs) that can potentially backfill from the internal carotid arteries (ICAs), acute occlusion of 1 VA is usually well tolerated by the vast majority of people, and the rate of cerebral ischemic complications in this setting is very low, in the range of 2–3 % [14]. The latter typically occur in people with a hypoplastic contralateral VA and small or hypoplastic PComAs. Likewise, partial injuries to 1 VA (e.g., dissection) with preservation of anterograde flow carries a potential risk of posterior circulation stroke secondary to thrombus formation at the site of injury and distal propagation or embolization into the vertebrobasilar system.
Zones of the Neck (Fig. 11.2)
From a penetrating trauma perspective, the neck has been traditionally divided into 3 anatomic zones, each with its specific vascular and visceral contents and relative ease of surgical accessibility. The location of the entry wound with respect to these anatomic zones is often used by trauma surgeons to guide acute management [21, 22].
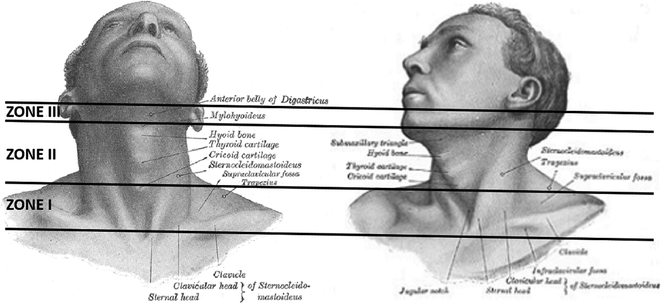

Fig. 11.2
Zones of the neck [modified from: Gray H, Lewis WH. Anatomy of the human body (20th ed). Philadelphia, PA: Lea & Febiger 1918]
Zone I extends from the sternal notch and clavicles inferiorly to the cricoid cartilage superiorly.
Zone II extends from the cricoid cartilage inferiorly to the angle of the mandible superiorly.
Zone III extends from the angle of the mandible inferiorly to the base of the skull superiorly.
In their course toward the skull, the VAs cross all 3 zones of the neck and, thus, can be virtually injured in penetrating trauma to any of these zones. In contrast to zone II, zones I and III are difficult to expose surgically (because of the sternum/clavicle head and mandible, respectively) and, thus, endovascular techniques are often preferred when managing unstable vascular injuries in those zones.
Patterns of VA Injury
The Denver classification system (Table 11.1) is often used to characterize blunt traumatic cerebrovascular injuries [3]. Although originally devised for blunt trauma, this classification system can also be easily applied to penetrating cerebrovascular injuries. In the setting of penetrating trauma , transection (grade V) and pseudoaneurysm formation (grade III) are particularly common injury patterns and may result in active hemorrhage (external bleeding, expanding neck hematoma, extravasation on CTA or angiography) or vertebral arteriovenous fistula (vertebral venous plexus or internal jugular vein). In one of the largest reported series from South Africa, the authors examined 92 penetrating VA injuries sustained in the civilian setting over a period of 16 years [15]. Among 88 patients who underwent angiography, they identified 39 VA occlusions, 36 pseudoaneurysms, 11 AVFs, and only 2 intimal injuries. Likewise, in a recent US military study of 11 penetrating VA injuries sustained in Iraq and Afghanistan over a period of 10 years, there were 5 pseudoaneurysms, 4 occlusions, 1 AVF, and 1 dissection [7].
Table 11.1
Denver classification system
Grade I: arterial dissection with less than 25 % luminal narrowing |
Grade II: arterial dissection with more than 25 % luminal narrowing |
Grade III: pseudoaneurysm |
Grade IV: occlusion |
Grade V: transection |
Associated Injuries
Associated injuries are common in the setting of penetrating neck trauma and are more likely to affect patient outcome than the VA injury itself [7, 12, 15, 28]. Bony injuries, including cervical spine and facial fractures, are observed in 10–70 % and are more common in the military setting, given the higher incidence of gunshot and blast injuries [7]. Likewise, spinal cord and nerve injuries (cranial nerves, brachial plexus) occur in 20–30 %, and are more common in combat-related trauma. Vascular injuries, both arterial (carotid vessels, axillary/brachial artery) and venous (vertebral venous plexus, internal jugular vein) are seen in approximately 15 %. Finally, aerodigestive tract injuries (esophagus, pharynx, larynx, trachea) affect roughly 10 % of patients.
Principles of Management
Initial management of patients with penetrating neck trauma follows the Advanced Trauma Life Support (ATLS) guidelines and largely depends on whether signs of active bleeding or hemodynamic instability are encountered in the primary survey [21].
Unstable Patients
Patients presenting with active hemorrhage from the neck (external bleeding or expanding hematoma) or hemodynamic instability should undergo emergent surgical exploration (for all zones, particularly zone II) and/or emergent angiography (for zones I and III) to identify and repair the injured vessel [21]. If a VA injury is encountered, reasonable efforts should be made to preserve the continuity of the vessel whenever possible. However, given the small caliber of the VA, the often complex pattern of injury, and the limited surgical access to its V2 segment, reconstructive surgical strategies (i.e., primary repair) are seldom successful and vessel sacrifice (i.e., surgical ligation) often becomes necessary. Conversely, emergency endovascular treatment, when available, offers the possibility of vessel preservation via placement of a covered stent graft across the injured VA segment or via stent-assisted coil embolization of a pseudoaneurysm or AVF [1, 10, 28]. Thus, when a hemorrhagic VA injury is encountered intraoperatively, surgical packing or tamponade with bone wax or a Fogarty balloon catheter (if bleeding from within the foramen transversarium) can be attempted, since it may provide temporary control of bleeding and allow a more definitive, potentially reconstructive, endovascular procedure (Fig. 11.3). It should be kept in mind, however, that stent-based endovascular reconstruction mandates dual antiplatelet therapy for several months after the procedure, to reduce the risk of in-stent thrombosis and secondary vertebrobasilar embolic stroke. Therefore, the hemorrhagic risks associated with dual antiplatelet therapy in the setting of a penetrating neck injury should be carefully considered when making the decision to stent an acutely injured VA.


Fig. 11.3
A 34-year-old man sustained a stab injury to the neck (zone II) using a knife. He presented with profuse bleeding from the neck and hemodynamic instability. Emergent surgical exploration of the neck revealed active hemorrhage from transection of the V1 segment of the right VA. Surgical ligation was attempted, but was unsuccessful. The wound was packed allowing temporary hemostasis and the patient was transferred emergently to the neurointerventional suite. a Right subclavian artery injection demonstrates occlusion of the right VA in its V1 segment, likely as a result of surgical packing. There is no evidence of contrast extravasation or distal collateralization of the VA from the ascending and deep cervical arteries. b The proximal stump of the VA was occluded with coils to achieve permanent hemostasis. c Right external carotid artery injection shows no evidence of distal collateralization of the right VA. d Left VA injection reveals excellent flow in the basilar artery and retrograde filling of the V4 and distal V3 segments of the occluded right VA. Given that no opacification whatsoever of the distal VA stump was demonstrated, distal control was deemed unnecessary. The patient was subsequently taken back to the operating room and the packing was successfully removed, without any evidence of residual active hemorrhage. He had an uneventful post-operative course and remained neurologically intact
When an artery cannot be reconstructed, both proximal and distal vascular control is generally required and can be accomplished either via direct surgical ligation or via endovascular embolization using platinum coils and/or liquid embolic agents (NBCA, Onyx). Fortunately, the rate of cerebral ischemic complications following acute unilateral VA occlusion is very low, in the range of 2–3 % [14]. In the endovascular setting, this can be further reduced by performing a complete cerebral and cervical angiogram prior to embolization, including the contralateral VA, bilateral ICAs, and ECAs, and bilateral thyrocervical and costocervical trunks (or subclavian arteries), to fully assess the extent of cervical and intracranial collaterals. In some cases, a balloon occlusion test of the injured VA can be performed prior to embolization, to confirm the presence of an adequate intracranial collateral circulation [10]. Endovascular access to the distal VA stump can be achieved either anterogradely via the proximal VA (in incomplete transections or lacerations) or retrogradely via the contralateral VA. However, distal vascular control is not always necessary after VA injury. In fact, in many cases, poorly developed distal cervical collaterals to the injured VA result in marginal retrograde flow and ultimately thrombosis and occlusion of its distal stump. Thus, if no significant retrograde angiographic filling or active bleeding is encountered from the distal VA stump, distal control can be safely omitted (Fig. 11.3). In the unlikely event of vertebrobasilar ischemia developing after unilateral VA occlusion, consideration may be given to surgical revascularization (e.g., ECA-VA bypass) at a later time.
Stable Patients
Patients with penetrating neck trauma who are hemodynamically stable should undergo a thorough physical examination followed by a CTA of the neck to determine the wound tract or trajectory and rule out underlying vascular or aerodigestive tract injuries [21, 22]. In the past, all zone II injuries were routinely surgically explored, given the relatively straightforward surgical access to that part of the neck. However, due to the high negative exploration rate encountered with this strategy, there has been a major paradigm shift over the past two decades, moving from mandatory neck exploration of all zone II injuries toward selective operative management based on the findings of CTA. Multidetector CTA has been shown to be a highly sensitive (90–100 %) and specific (90–100 %) imaging modality for traumatic vascular and visceral injuries. However, when bone fragments or metallic foreign bodies overlie the VA, beam-hardening artifacts may result in suboptimal visualization of the vessel. Thus, when CTA is inconclusive or doubt persists regarding a possible VA injury despite a negative CTA (e.g., wound trajectory crosses VA), catheter angiography should be performed, as it remains the gold standard for vascular imaging.
The optimal management of hemodynamically stable penetrating VA injuries remains largely uncertain, given the rarity of these injuries, resultant paucity of the literature, marked only by a few retrospective case series of small sample sizes [1, 7, 9, 10, 12, 15, 28], and their unknown natural history. Treatment of stable penetrating VA injuries usually varies with the type and severity of injury. In general, endovascular treatment is usually preferred over surgery, given its lower risk of complications, high technical success rate, and the possibility of vessel preservation [1, 10, 28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





