Chapter 12 Visual Disturbances
This chapter describes several common visual disturbances likely to occur in psychiatric patients, including decreased visual acuity, glaucoma, visual field loss, and visual hallucinations (Box 12-1). It includes several causes of visual impairment in the elderly (Box 12-2). Whatever the cause of a visual disturbance, it will probably carry psychiatric comorbidity.
Box 12-1
Common Neurologic Causes of Visual Hallucinations
*Although visual hallucinations are likely to complicate almost any form of dementia, they are characteristic of dementia with Lewy bodies disease.
†Antiparkinson medications, such as levodopa-carbidopa (Sinemet), are more likely than Parkinson disease itself to produce hallucinations.
Evaluating Visual Disturbances
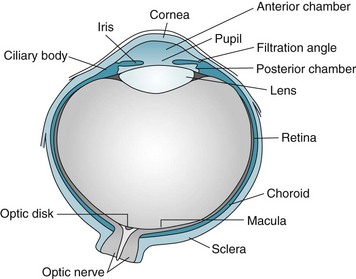
After determining the patient’s specific visual symptom, the physician’s initial examination typically includes inspecting the globe or “eyeball” (Fig. 12-1) and eyelids; assessing visual acuity, visual fields, and optic fundi; and testing pupil reflexes and ocular movement. Physicians must perform additional examinations for psychogenic blindness, visual agnosia, and other perceptual disturbances.
Physicians routinely measure visual acuity by having the patient read from either a Snellen wall chart or a hand-held card (Fig. 12-2). A person with “normal” visual acuity can read 3/8-inch (0.6-cm) letters at a distance of 20 feet (6.1 meters). This acuity, the conventional reference point, is designated 20/20. People with 20/40 acuity must be as close as 20 feet (6.1 meters) to see what a person with normal acuity can see at a distance of 40 feet (12.2 meters).
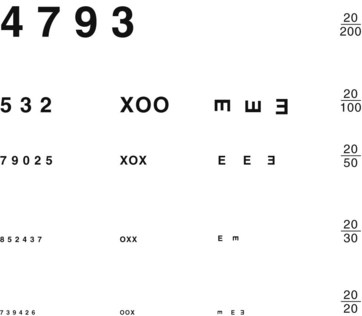
FIGURE 12-2 Physicians should hold this hand-held visual acuity chart 14 inches (36 cm) from patients and test each individual eye. The smallest line that they read without a mistake determines their visual acuity. For neurologic evaluations, patients should wear their glasses or contact lenses.
Optical Disturbances
The usual causes of myopia are optical rather than neurologic, such as a lens that is too “thick” or a globe that is too “long” (Fig. 12-3). Occasionally medicines cause myopia. For example, topiramate (Topamax), a widely prescribed antimigraine and antiepileptic drug (AED), may produce an acutely occurring but transient myopia. (Topiramate can also lead to angle closure glaucoma [see later].)
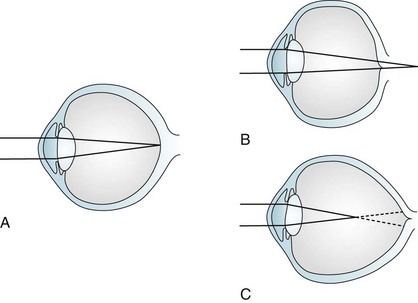
FIGURE 12-3 Image focusing in hyperopic (farsighted) and myopic (nearsighted) eyes. A, In normal eyes, the lens focuses the image on to the retina. B, In hyperopic eyes, the shorter globe or improperly focusing lens causes the image to fall behind the retina. C, In myopic eyes, the longer globe or improperly focusing lens causes the image to fall in front of the retina. Lenses can compensate for the refractive errors of hyperopia and myopia. Alternatively, laser or surgical “flattening” of the lens may correct myopia.
Because the parasympathetic nervous system mediates the accommodation reflex, many medications with anticholinergic side effects impair visual acuity for closely held objects (Fig. 12-4). These medicines – selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, and clozapine – produce blurred vision mostly because their substantial anticholinergic properties impair patients’ accommodation reflex. For example, duloxetine (Cymbalta) causes blurred vision in approximately 3% of patients; sertraline (Zoloft) and paroxetine (Paxil), 4%; and venlafaxine (Effexor) at 75 mg, 9%. This side effect may be unsuspected because these medicines can impair accommodation without producing other anticholinergic effects, such as dry mouth, constipation, and urinary hesitancy.
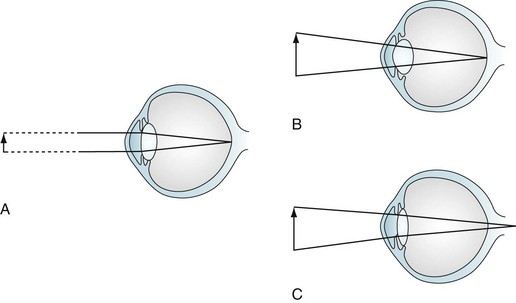
FIGURE 12-4 Accommodation and accommodation paresis. A, When looking at a distant object, parallel light rays are refracted little by a relatively flat lens on to the retina. B, Accommodation: when looking at a closely held object, ciliary muscle contraction increases the curvature of the lens, greatly refracting the light rays. C, Accommodation paresis: If the ciliary muscles are paretic, the lens cannot form a rounded shape. Its weakened refractive power focuses light rays from closely held objects behind the retina; however, parallel light rays from distant objects still focus on the retina. Therefore, accommodation paralysis blurs closely held objects but leaves distant ones distinct.
Abnormalities of the Lens, Retina, and Optic Nerve
Cataracts (loss of lens transparency) result from complications of old age (senile cataract), trauma, diabetes, myotonic dystrophy (see Chapter 6), and chronic use of certain medicines, such as steroids. In prolonged, high doses, phenothiazines and some second-generation neuroleptics may produce minute lens opacities, but ones rarely dense enough to impair vision.
Pigmentary changes in the retina can be a manifestation of injuries, degenerative diseases, diabetes, infection, or the use of massive doses of phenothiazines (Fig. 12-5). Among infants and children, nonaccidental head injury (child abuse), particularly violent head shaking or direct trauma, creates retinal hemorrhages. Other stigmata of repeated trauma – spiral fractures of the long bones, multiple skull fractures, and burns (see Chapter 22) – frequently accompany these retinal hemorrhages.
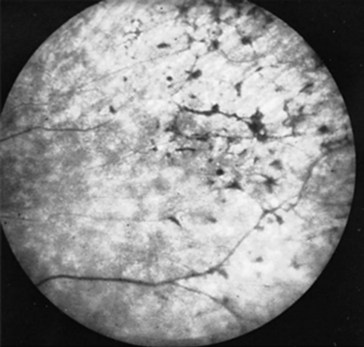
FIGURE 12-5 Massive doses of thioridazine (Mellaril) may induce retinal hyperpigmentation – described as “black bone spicules” or “salt and pepper.” Before these retinal pigmentary changes are visible on fundoscopic examination, patients may complain of blurred vision or impaired nighttime vision.
In 25% or more of Americans older than 65 years, the cells of the retina’s pigment epithelium, mostly in the macula, degenerate through a variety of mechanisms, including proliferation of the underlying blood vessels. When degeneration involves cells in the macula, a condition known as macular degeneration, it distorts patients’ critical central vision. Patients characteristically lose their reading ability. With their remaining peripheral vision, patients negotiate around their living areas. However, progressive deterioration deprives patients of all their eyesight. As with individuals who develop blindness from any cause, those beset by macular degeneration are at risk of losing their self-sufficiency, appearing to have cognitive dysfunction, and experiencing visual hallucinations (especially if they also have hearing or cognitive impairments [Box 12-1]).
Optic Nerve
Injuries of the optic nerves, which are projections of the central nervous system (CNS), result in visual loss that may be limited to a scotoma (an area of blindness [see Fig. 15-2]), but may encompass the entire visual field. In addition, because the optic nerves serve as the afferent limb of the light reflex, optic nerve injury also causes an afferent pupillary defect: When the examiner shines light into an eye with optic neuritis, both pupils fail to constrict; however, when the same light shines into the unaffected eye, both pupils normally constrict (see Fig. 4-2). With time, optic nerve injuries usually cause atrophy, revealed by the fundoscopic examination showing pallor of the optic head.
Optic nerve injuries may occur either as isolated conditions or ones accompanied by injuries of the cerebrum or other part of the CNS. One of the most common, inflammation of one or both optic nerves, optic or retrobulbar neuritis, causes sudden, painful visual loss (Fig. 12-6), as well as an afferent pupillary defect. In addition, they see “desaturated” colors with their remaining vision. For example, patients cannot appreciate the difference between fire engine red and brick red. In severe cases, they cannot distinguish red from green.
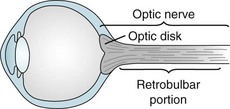
FIGURE 12-6 Neurologists consider the optic disk, which they can see on fundoscopic examination, to be the bulbar portion of the optic nerve. They consider the long segment of the optic nerve behind the eye its retrobulbar portion. Depending on where an inflammatory condition, such as multiple sclerosis, attacks the optic nerve, patients develop optic or retrobulbar neuritis. Visual loss, pain on eye movement, and color desaturation characterize optic and retrobulbar neuritis.
Of the many conditions that cause optic neuritis, demyelinating illnesses, particularly multiple sclerosis (MS) and its close relative neuromyelitis optica (NMO), are the most common (see Chapter 15). Most importantly, optic neuritis frequently precedes the appearance of other manifestations of MS and complicates the course of most cases. If an otherwise asymptomatic patient develops optic neuritis and the magnetic resonance imaging (MRI) shows two or more hyperintense lesions in the brain, that patient has greater than a 70% risk of developing MS. On the other hand, an otherwise asymptomatic optic neuritis patient who has no MRI lesions has only a 25% risk of developing MS.
An inflammatory condition of the arteries that supply the optic nerve, temporal or giant cell arteritis, often leads to ischemia of the optic nerves. Moreover, the arteritis tends to spread to the cerebral arteries (see Chapter 9). Typically affecting only people older than 65 years, temporal arteritis often first causes a mild to moderate, prolonged (weeks to months) illness featuring headache accompanied by systemic symptoms, such as malaise, prolonged aches, and pains. The number and variety of these initial nonspecific symptoms understandably lend the appearance of depression or a somatoform disorder. However, physicians should avoid missing this diagnosis because, unless they promptly treat the patient with high-dose steroids, it can result in blindness and strokes. Finding giant cells and other signs of inflammation on a temporal artery biopsy will confirm the diagnosis.
Leber hereditary optic atrophy, an illness attributable to a mitochondrial DNA mutation, also involves the optic nerves, but no other part of the CNS or the musculature (see Chapter 6). Most commonly affecting young males, it causes visual loss culminating in blindness in one and then, within months, the other eye.
Another classic example of simultaneous injury of both the optic nerve and cerebrum is an olfactory groove or sphenoid wing meningioma. This tumor compresses the near-by optic nerve (see Chapters 19 and 20) and burrows into the overlying frontal or temporal lobe. The cerebral damage can trigger complex partial seizures and cause cognitive decline and personality changes. At the same time, optic nerve damage causes optic atrophy and blindness in one eye.
Similarly, tumors of the pituitary region, such as adenomas or craniopharyngiomas, may also produce visual loss accompanied by psychologic changes. Unless detected and removed early, these tumors grow slowly upward to compress the optic chiasm and hypothalamus and downward to infiltrate the pituitary gland (see Fig. 19-4). Compression of the optic chiasm causes the pathognomonic bitemporal hemianopsia. Compression of the hypothalamus and pituitary causes headache and panhypopituitarism: decreased libido, diabetes insipidus, loss of secondary sexual characteristics, and sleep disturbances.
Glaucoma
In most cases, glaucoma consists of elevated intraocular pressure resulting from obstructed outflow of aqueous humor through the filtration angle of the anterior chamber of the eye (Fig. 12-7) – not from increased production of aqueous humor. Psychiatrists should recognize two common varieties – open-angle and angle closure – although only certain psychotropic medications occasionally produce the angle closure variety. If glaucoma remains untreated, it damages the optic nerve, causes visual field impairments, and eventually leads to blindness.
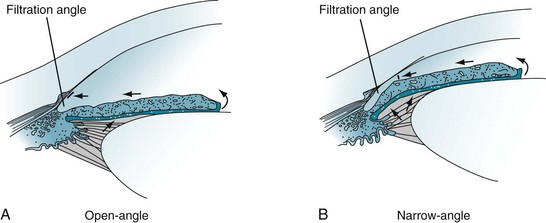
FIGURE 12-7 A, Open-angle glaucoma: The aqueous humor does not drain despite access to the absorptive surface of the angle. Impaired flow from the eye leads to gradually increased intraocular pressure (glaucoma). B, Narrow-angle glaucoma: When the iris moves forward, as may occur during pupil dilation, the angle is narrowed or even closed. Obstruction of aqueous humor flow leads to angle closure glaucoma.
Angle Closure Glaucoma
In angle closure glaucoma, which is also called closed-angle or narrow-angle glaucoma, intraocular pressure is usually elevated by impaired aqueous humor outflow at the filtration angle (see Fig. 12-7). In one variety, the fluid becomes trapped behind the iris. Patients with narrow-angle glaucoma usually are older than 40 years and often have a family history of the disorder, but they also frequently have a history of hyperopia and long-standing narrow angles. Few have had symptoms, such as seeing halos around lights, preceding an attack of angle closure glaucoma. In contrast to the relatively normal appearance of the eye in open-angle glaucoma, in acute angle closure glaucoma the eye is red, the pupil dilated and unreactive, and the cornea hazy. Moreover, the eye and forehead are painful, and vision is impaired.
Angle closure glaucoma is sometimes iatrogenic. For example, when pupils are dilated for ocular examinations, the “bunched-up” iris can block the angle (see Fig. 12-7, B). Likewise, medicines with anticholinergic properties, probably because they dilate the pupil, can precipitate angle closure glaucoma.
Cortical Blindness
Bilateral occipital cortex injuries can produce severe visual impairment, called cortical blindness. The underlying cause may be damage limited to the occipital lobes from bilateral posterior cerebral artery occlusions or trauma. Alternatively, extensive brain injury from anoxia, multiple strokes, or MS may cause cortical blindness along with other impairments. Reflecting occipital lobe damage, electroencephalograms (EEGs) characteristically lose their normal, posterior 8–12-Hz (alpha) rhythm. Whether the cortical blindness results from limited or generalized cortex injury, the pupils are normal in size and reactivity to light because all elements of the pupillary light reflex remain intact: the midbrain and optic and oculomotor nerves (see Fig. 4-2).
Anton Syndrome
The dramatic neuropsychologic phenomenon of Anton syndrome – blind patients explicitly or implicitly denying that they have lost all vision – characteristically complicates the sudden onset of blindness. Whether the blindness stems from a blast injury of both eyes, bilateral occipital lobe strokes, or other cause, the irrational response to blindness, rather than blindness itself, constitutes Anton syndrome. These patients typically respond, as those with anosognosia (see Chapter 8), by using denial as a defense. Sometimes they simply refuse to say that they have lost vision. Others blame external factors, like dim light, for their problem. Some may, if pressed, acknowledge visual loss but confabulate by “describing” their room, clothing, and various other objects. Anton syndrome allows blind patients to behave as though they still had normal vision and proceed to stumble about their room.
Visual Perceptual Disturbances
Agnosia
Visual agnosia is also a major component of the infamous, although uncommon, Klüver–Bucy syndrome. Neurosurgeons have produced this behavioral disorder in monkeys by resection of both anterior temporal lobes, which contain the amygdalae and components of the limbic system. The resulting limbic system damage produces visual agnosia so severe that the monkeys not only touch all objects, but they compulsively identify all objects by putting them into their mouth (“psychic blindness”). Their behavior can be repetitive, compulsive, and indiscriminate. When the Klüver–Bucy syndrome occurs in humans (see Chapter 16), they display a muted variation of psychic blindness, oral exploration, which consists of their placing inedible objects in their mouth, although only briefly, partly, and absent-mindedly.
Psychogenic Blindness
Similarly, tubular or tunnel vision defies the laws of optics that dictate that the visual area should expand with increasing distance (Fig. 12-8). Important exceptions to this general rule, however, sometimes occur when patients with migraine with aura have constriction of their peripheral vision and in some patients taking vigabatrin.
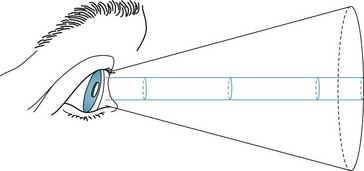
FIGURE 12-8 The area seen by a person normally increases conically in proportion to the distance from the object. In tubular or tunnel vision, which defies the laws of optics, the “visible” area remains constant despite increasing distance.
If clinical tests are inconclusive, EEG and other electrophysiologic testing may help. Alpha rhythm overlying the occipital lobes of patients at rest with their eyes closed, and loss of that rhythm when they open their eyes, indicates an intact visual system. However, because patients’ anxiety or concentration suppresses alpha activity, its absence is not as meaningful as its presence. In visual-evoked response testing, another noninvasive electrophysiologic test, visual system injuries produce abnormal potentials (see Chapter 15).
Visual Hallucinations
Visual hallucinations can originate in dysfunction of the frontal, temporal, or occipital cortex. Problems as diverse as toxic-metabolic encephalopathy, dementia-producing diseases, medication side effects, and structural lesions can produce them (see Box 12-1).
Seizures
Elementary partial, complex partial, or frontal lobe seizures (see Chapter 10) can produce visual hallucinations. Seizure-induced visual hallucinations tend to be stereotyped and brief, can be “seen” in both eyes, and may even appear in a hemianopic area. They range from simple geometric forms in elementary partial seizures to detailed visions accompanied by sounds, smells, thoughts, emotions, and, characteristically, impairment of consciousness, in complex partial seizures.
Migraine Aura
The “aura” in migraine with aura (previous termed “classic migraine”) consists of sensory disturbances – olfactory, sensory, or visual. In almost all cases, auras include stereotyped visual hallucinations (see Fig. 9-2). The majority consist of distinctive crescent scotomata or scintillating, patterned zig-zag lines (fortification spectra) that move slowly across the visual field for 1–20 minutes before yielding to a hemicranial headache. In a potentially confusing situation, visual auras sometimes represent the sole manifestation of migraine. In rare individuals, migraine aura consists of elaborate visual distortions, such as metamorphopsia, in which individuals and objects appear, to the patient, to change size or shape, as in the celebrated Alice in Wonderland syndrome.
Narcolepsy
As an element of the narcoleptic triad (see Chapter 17), visual hallucinations intrude into a patient’s partial consciousness. Narcoleptic-induced visual hallucinations are essentially dreams composed of variable, unpredictable – not stereotyped – intricate visions accompanied by rich thoughts and strong emotions. They tend to occur while patients fall asleep (hypnagogic hallucinations) or awaken (hypnopompic hallucinations). As with normal dreams, these hallucinations are associated with flaccid, areflexic paresis and rapid eye movements (REMs).
Neurodegenerative Illnesses
Visual hallucinations are also a hallmark of neurodegenerative diseases that cause dementia, particularly Alzheimer, Lewy bodies, and Parkinson diseases or their treatment (see Chapters 7 and 18). When they are manifestations of these disorders, hallucinations tend to be visually complex, have a paranoid aspect, and occur predominantly at night. As a clue to dementia with Lewy bodies disease, visual hallucinations occur frequently and begin early in its course. In contrast, when visual hallucinations complicate Alzheimer disease, they occur in its late stages. Hallucinations in Parkinson disease are usually partly medication-induced.