8
8 
What Is the Safest Way to Prevent Deep Venous Thrombosis and Pulmonary Embolism After Head or Spinal Cord Injury? How Soon After Surgery or Injury Can I Anticoagulate My Patients Who Develop Deep Venous Thrombosis?
What is the Safest Way to Prevent Deep Venous Thrombosis and Pulmonary Embolism After Head or Spinal Cord Injury?
BRIEF ANSWER
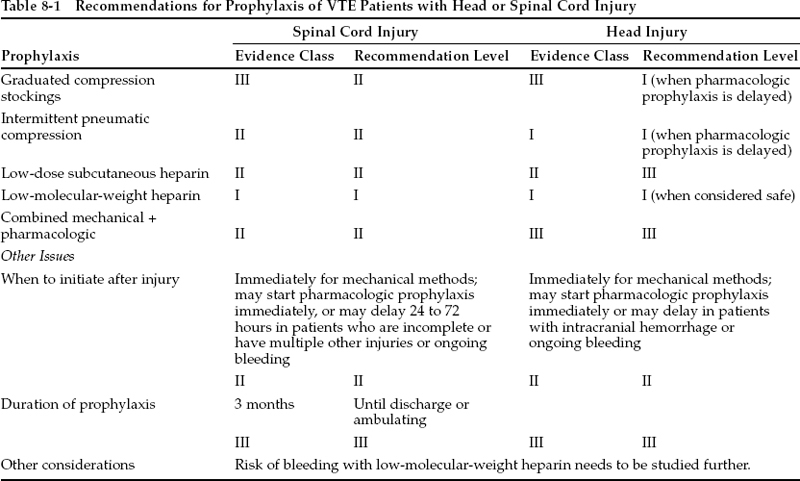
For patients with spinal cord injury, low-molecularweight heparin (LMWH) is the recommended method of deep venous thrombosis (DVT) prophylaxis. This treatment should be delayed for 24 to 72 hours in patients with incomplete injuries. Treatment should continue for 3 months, perhaps with more aggressive anticoagulation after 2 weeks. Thigh-high intermittent pneumatic compression (IPC) may be instituted immediately and may be used in conjunction with pharmacologic measures. For head-injured patients, IPC is the initial method of choice for DVT prophylaxis. If desired, LMWH can probably be used in place of IPC after the first 2 or 3 days. Treatment should probably be continued until discharge to home or to rehabilitation. Prophylactic use of inferior vena cava filters is not recommended. These recommendations may need to be modified for patients with lower extremity injuries, coagulopathy, anticipated future surgery or invasive procedures, etc.
Background
The goals of venous thromboembolism (VTE) prophylaxis are to prevent complications of pulmonary embolism (PE) (especially death) and to prevent the complications of DVT, the most important being the postphlebitic syndrome. Two different approaches have been advocated. One is to screen patients for subclinical DVT and institute therapy whenever DVT is detected. The second is primary prophylaxis to prevent development of VTE. Primary prophylaxis is the most viable option at this time because it is more effective and less expensive and because screening tests such as duplex ultrasound have only moderate sensitivity and positive predictive value for detecting DVT in asymptomatic, high-risk patients.1 It must be emphasized that clinical features of DVT are useless as screening tools. Only three of 201 trauma patients (1.5%) with venogram-proven DVT had clinical signs or symptoms before venography (class II data).2 These figures likely hold true for other patient populations.
Risk Factors
Risk factors for VTE can be classified as a triad, described by Virchow as abnormalities in blood coagulation, in blood flow, and in the vein wall. Common risk factors are increasing age, obesity, varicose veins, immobility, paresis or plegia of the limbs, systemic infection, pregnancy and the puerperium, estrogen therapy, previous VTE, malignancy, deficiency of antithrombin 3, protein C or protein S, activated protein C resistance (factor 5 Leiden), prothrombin variant 20210A, lupus anticoagulant, antiphospholipid antibody syndrome, heparin-induced thrombocytopenia, trauma (especially spinal cord injury and fractures of the pelvis and lower limb), heart failure, systemic infection, indwelling central venous catheters, and surgery.
In patients undergoing surgery, the risk is higher with certain types of surgery. Intracranial surgery has a greater risk than spinal surgery, and major orthopedic surgery of the lower limbs and of the pelvis has a high risk that increases with the number of fractures repaired. Risk increases with longer times of anesthesia, with general anesthesia as compared with spinal or local anesthesia, and with postoperative patient immobilization.3
The risk of VTE can be estimated from these factors. This is important since prophylaxis itself may carry risk, which has to be balanced against the risk that a patient will develop VTE. Low-risk patients include those with isolated spinal fractures but not cord injury. They have a risk of calf DVT of <10%. Moderate-risk patients have a calf DVT risk of 10 to 40% and include patients with isolated lower limb fractures who are under 40 years old. High-risk patients have a calf DVT risk of 40 to 80% and include most patients with spinal cord injury, multiple trauma, elective intracranial neurosurgery, major orthopedic surgery of the lower limbs, or prior VTE.
Methods of DVT Prophylaxis
Prophylaxis for prevention of VTE may be mechanical and/or pharmacologic. Mechanical methods include graduated compression stockings, IPC of the lower limbs (usually the calves), rotating tables, and electrical stimulation of the calf muscles. Pharmacologic methods include low-dose unfractionated heparin, LMWH, adjusted-dose heparin, or warfarin.
In general, the greater the degree of pharmacologic anticoagulation that is achieved, the lower the risk of VTE and the higher the risk of bleeding. Meta-analysis of numerous large randomized clinical trials suggests that there is no increase (or only a small increase) in wound bleeding in patients treated with low-dose unfractionated heparin or LMWH.1 This is of more concern in neurosurgical patients than other surgical patients and is carefully considered in the recommendations that are made in this chapter (Table 8-1). Mechanical methods of prophylaxis avoid this risk but may be impractical in many trauma patients because of lower limb injuries. It has been suggested that a systemic fibrinolytic state can be induced by intermittent compression even of the upper limbs or feet, but efficacy has not been proven in randomized trials. Other limitations of mechanical methods are that they may not be as efficacious in high-risk patients or in routine practice, where compliance may be lower than in a clinical trial.
It is recommended that pharmacologic prophylaxis be withheld the morning of surgery and resumed 24 hours later in the absence of ongoing bleeding. Patients who are exposed to heparin in any dose, even as a flush in intravascular catheters, should have their platelet count measured every 2 days or so for the first 14 days of exposure to detect heparin-induced thrombocytopenia. This complication is less common in patients receiving LMWH.
Pearl
Patients with any exposure to heparin (even as a flush in intravascular catheters) should have their platelet count measured frequently to detect heparin-induced thrombocytopenia. This occurs less commonly in patients receiving LMWH.
Data reviewed below are from randomized trials in trauma and spinal cord injury patients. If no such trials have been conducted, prospectively and retrospectively collected data are reviewed. The neurosurgical literature contains five trials of mechanical and seven of pharmacological prophylaxis, but trauma patients were entered into only one, which showed lack of efficacy of an antiplatelet agent.
Literature Review: Spinal Cord Injury
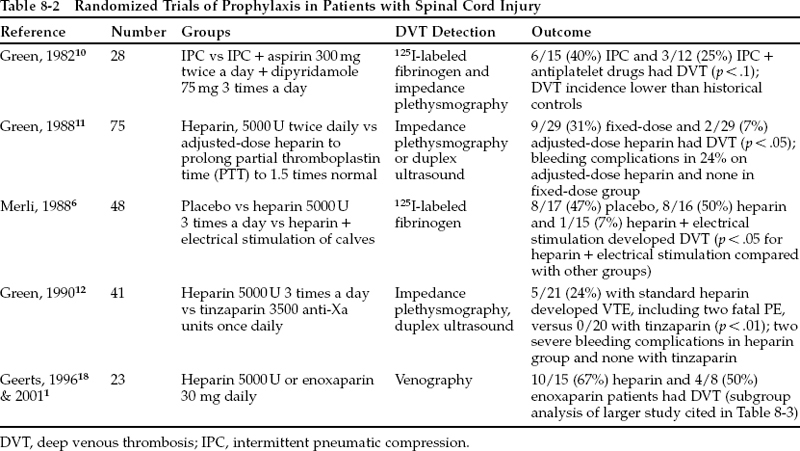
Spinal cord injury carries one of the highest risks of VTE. A multicenter study of 1419 patients with acute spinal cord injury noted that 15% developed symptomatic DVT and 5% developed clinically evident PE (class III data).4 The incidence of DVT in observational studies and in the untreated control groups of randomized trials of patients studied acutely after spinal cord injury was 71% [45/63 patients in five trials (class I—III data), range 47% to 100%] as detected by125 I-labeled fibrinogen5–9 and 81% (21/26 patients in one trial) as detected by venography (class I data).2
Pearl
Antiplatelet drugs are generally not efficacious for VTE prophylaxis, and their use for this purpose is not recommended.
Randomized trials have been conducted in patients with spinal cord injury (Table 8-2). Interpretation is hampered by small numbers of patients, lack of inclusion of placebo groups, lack of venography as an end point, and use of many different regimens. A randomized comparison of IPC alone versus IPC plus aspirin and dipyridamole noted no significant difference between the groups (class I data).10 Antiplatelet drugs are generally not efficacious for VTE prophylaxis in any setting, and their use is not recommended. Retrospective and prospective observational studies of patients treated with low-dose heparin yield conflicting results. They seem to suggest that heparin only slightly reduced the risk of DVT, with no increase in bleeding.1 Comparison of low-dose heparin to placebo and to heparin plus electrical stimulation of the calf muscles found that heparin alone was ineffective but that combined treatment was beneficial.6 Since low-dose heparin alone seemed inadequate, adjusted-dose heparin administered to elevate the partial thromboplastin time to 1.5 times normal was compared with low-dose heparin and was found to be more effective, but with a significant increase in bleeding complications (class I data).11 A randomized trial comparing the LMWH tinzaparin to heparin noted that tinzaparin significantly reduced DVT without increasing bleeding (class I data).12 Uncontrolled data from 60 selected patients treated with tinzaparin for 8 weeks found an incidence of DVT of 18% and bleeding in 3% (class III data).13 Patients with head injury, hemothorax, and long bone fractures were excluded. In another report, enoxaparin prophylaxis in 105 patients was associated with a 10% risk of bleeding and with no DVTs in the 60 patients screened with ultrasound (class II data).14
Pearl