 23
23 
Why Have Therapeutic Trials in Head Injury Been Unable to Demonstrate Benefits?
The topic of this chapter does not lend itself to the class I, II, or III format for the analysis of published studies.
BRIEF ANSWER
The Decade of the Brain saw several dramatic advances in our understanding of the pathophysiology of traumatic brain injury (TBI) and focal and global cerebral ischemia. Unfortunately, however, with the exception of tissue plasminogen activator (tPA) for ischemic stroke, these advances in our understanding of the biologic mechanisms that produce brain damage have not translated into success in the clinical arena. Reasons for our failure to improve the outcomes of our patients are multifactorial and include the following:
- Failure to adequately test therapeutic agents in animal models
- Presence of multiple mechanisms of brain damage at the cellular level
- Complexity of head injury, making it unlikely that any single treatment will be efficacious, and therefore requiring either drugs with multiple mechanisms of action or sequential therapy with different drugs
- Inappropriate mechanism of action of investigational drugs in terms of the types of injuries in patients selected for clinical trials
- Incomplete understanding of drug mechanism of action, with the result that reasons for failure of clinical trials were incorrectly assigned
- Inadequate understanding of required duration of treatment
- Lack of sufficiently sensitive measures to detect favorable changes in neurobehavioral outcome
The complexity of brain injury is a consequence of a myriad of neurochemical processes that begin after the damage of the initial injury. Unlike stroke, in which the damage is initiated some time after the initial ictus, much of the damage in head injury (perhaps more than we would like to admit) occurs at the time of impact, particularly in more severe injuries. A chain of neurochemical events is then superimposed upon an already damaged brain. Coupled with the brain’s unique and still very poorly understood vulnerability to ischemia, the incredible complexity and dynamic nature of brain injury have thus far thwarted our overly simplistic therapeutic initiatives.
Background
Phase I trials attempt to establish the safety of the maximum tolerated dose of a drug. Because neuroprotective agents are often tested earlier in patients with stroke or subarachnoid hemorrhage (SAH), phase I trials are not always conducted in head-injured patients. A major weakness of this strategy is the fact that dose-limiting side effects in patients without head injury (such as depressed consciousness, depressed respiration, and development of hallucinations) may be irrelevant in head-injured patients, who may be comatose or mechanically ventilated. Phase II trials are blinded and enroll 25 to 50 randomized patients per group to demonstrate preliminary efficacy. Phase II findings enable power calculations for later phase III trials. Phase I and phase II studies have defined entry criteria, an outcome measure (typically clinical recovery for phase II studies), and multiple secondary end points. Phase III trials are also randomized but, in addition, are placebo-controlled and double-blind. They often include at least 400 patients per group to test whether a drug improves outcome over the current standard of care or over placebo.
The main objective of the treatment of patients with head injury is prevention of secondary damage. Causes of secondary damage include systemic insults such as hypotension and hypoxia, delayed or recurrent intracranial mass lesions, and biochemical processes leading to cell membrane dysfunction and disturbed circulation in the microvasculature. Appropriately, recent clinical trials have involved several agents believed to be neuroprotective against these putative mechanisms of secondary damage. For reasons outlined above, however, none of the 10 published reports of clinical trials performed during the past decade has proven any agent to be efficacious in the general head injury population. Of the 10 studies, four were terminated early, and only six were actually completed.
Pearl
A drug’s dose-limiting side effects in patients without head injury may be irrelevant in comatose or mechanically ventilated head-injured patients.
Literature Review of Head Injury Trials
Corticosteroids
Although corticosteroids have a known role in controlling tumor-related edema, no clear indication for their use in head injury has been identified.1 Several prospective randomized clinical trials have failed to show that corticosteroid use was associated with statistically significant improvement in outcome for the general study population.2 In the triamcinolone study, however, a statistically significant effect was noted in a subgroup of patients with focal lesions and Glasgow Coma Scale scores (GCS) <8 on admission.3 A metaanalysis of both published and unpublished trials of corticosteroids in head injury showed a possible 2% reduction in mortality and also determined that a prospective trial of 20,000 would be necessary to confirm or exclude this possibility.2 The Corticosteroid Randomization After Significant Head Injury (CRASH) Trial is now attempting to test the efficacy of corticosteroids in an appropriately sized sample.4,5
Pearl
Treatment of head injury with corticosteroids may be associated with a 2% reduction in mortality.
Calcium Channel Antagonists
Nimodipine is known to reduce the risk of ischemia after spontaneous SAH6 but has not yet been shown to be effective in traumatic SAH. The British/Finnish Cooperative Head Injury Trial Group study (HIT I) of 351 patients with severe head injury showed an 8% relative but statistically insignificant improvement in 6month outcome.7 The HIT II study was a prospective, multicenter, placebo-controlled trial of 852 patients with severe head injury. Again, nimodipine showed a relative but statistically insignificant increase in favorable outcome and decrease in unfavorable outcome for the overall population.8 Nimodipine did, however, significantly reduce unfavorable outcome in a subgroup with traumatic SAH. The HIT III trial was a prospective, multicenter, randomized trial of 123 patients with traumatic SAH. Although a statistically significant effect was noted,9 the finding was not accepted universally because the presence of SAH could not be reconfirmed later in one fifth of patients.
Analysis of pooled data from all three trials shows a significant benefit from nimodipine in traumatic SAH. However, given the different inclusion criteria for the three trials, the validity of such a result is questionable. Furthermore, a retrospective analysis of HIT I patients with traumatic SAH did not show nimodipine to be protective.10 Results from the HIT IV trial, which was focused on the efficacy of nimodipine for traumatic SAH, showed no effect of the drug in this targeted population.11
Free Radical Scavengers
Tirilazad mesylate is an inhibitor of free radical– mediated lipid peroxidation.12 In phase I and phase II trials, it was shown to have a good safety profile and to be effective in experimental models of head injury even when hypoxia was present. Two prospective multicenter trials were then launched to study its efficacy. The North American study was terminated before full enrollment was attained because of higher mortality in the treatment group, although this increase was found not to be significant when 6-month outcomes were analyzed.13 The European/Australian trial included 957 patients with severe head injury and 163 with moderate head injury; a statistically significant effect in the full population was not demonstrated, though a significant effect in males with traumatic SAH was noted.14
Polyethylene glycol-conjugated superoxide dismutase (PEG-SOD) is another free radical scavenger that significantly improved outcome in phase II studies. Two consecutive phase III trials were initiated in patients with severe head injury. Although the treatment group had a more favorable outcome profile, outcomes in the two groups were not significantly different.15 Improved trial design may have improved the chances of attaining significance in a subsequent trial, but the sponsoring American company was bought out by a French company that had little interest in pursuing studies of a drug extracted from cow liver because of fear of potential transmission of bovine spongiform encephalopathy (” mad cow disease“).
N-Methyl-D-Aspartate (NMDA)/Glutamate Receptor Antagonists
The competitive NMDA receptor antagonist Selfotel (CGS 19755) was tested in two multicenter phase III trials of patients with severe head injury16 after investigators reported that excessive concentrations of excitatory amino acids, especially glutamate, can lead to secondary damage17 and, further, that NMDA antagonists can block ischemia-induced neuronal degeneration.18,19 Before full enrollment was attained, however, the head injury trials were stopped because of concern over increased deaths and serious adverse events in the treatment groups of two concurrent stroke trials.20 Though the head injury data did not show an increased frequency of serious adverse events, the data suggested that efficacy could not be demonstrated even if the trials were allowed to continue to completion. Subsequent studies revealed that up to 40% of patients enrolled in these trials had no intracranial mass lesions, such as focal contusions and acute subdural hematomas, and therefore constituted groups with a low likelihood of excess glutamate release as part of their pathophysiology.21,22
The noncompetitive NMDA receptor antagonist Cerestat (CNS 1102) was also tested in a phase III trial of patients with severe head injury after it was found to exhibit neuroprotection in laboratory models of ischemic brain injury and head trauma. In animals with cortical impact injury, Cerestat reduced contusion volume, decreased hemispheric swelling and water content, lowered intracranial pressure, and improved cerebral perfusion pressure. During the phase III trial in head-injured patients, concerns about the effects of Cerestat in stroke patients and about the futility of continuing the trial led to the termination of the study before full enrollment was attained.22 However, subsequent analysis suggested that a subset of stroke patients may in fact have benefited from Cerestat.
Pearl
Although interim analyses in stroke trials have raised concerns about potential adverse effects of NMDAreceptor antagonists, such concerns do not seem to have been justified in head injury trials.
The confidence intervals for the aforementioned trials are plotted in Fig. 23-1. Although it can be argued that the neuroprotective agents in general appear to be somewhat efficacious in the total population of head-injured patients, a beneficial effect is much more obvious in the subgroup of patients with traumatic SAH or focal contusions. A meta-analysis of the previous trials to estimate the pooled effect of the neuroprotective agents in the total population of head-injured patients yielded an odds ratio of 0.9 (95% confidence interval 0.70–1.06).22
Recommendations Based on Lessons Learned from Head Injury Trials
Perform Adequate Preclinical Studies
Before phase III trials are initiated, investigational agents must undergo adequate preclinical testing to answer key questions. Conclusions about dose– response curves, time window response curves, length of treatment, therapeutic range, target organ drug availability, and drug metabolism must be accurately extrapolated from experimental models. Specifically, the tirilazad and Selfotel examples demonstrate the importance of phase I and II studies for characterizing pharmacokinetics and hence target organ drug availability, for identifying the appropriate group of patients in whom pathophysiology matches the drug’s mechanism of action, and for demonstrating safety. Only after termination of the tirilazad and PEG-SOD trials was it recognized that both agents fail to demonstrate significant crossing of the blood—brain barrier (BBB) after severe head injury.14,15 It was also discovered that women had lower plasma levels of tirilazad than men, sometimes below the therapeutic range,23 and that metabolism of tirilazad was increased by phenytoin.24 This latter issue is of concern because seizure prophylaxis with phenytoin is common in head injury management in the United States. The fact that tirilazad was shown to have significant efficacy in men with traumatic SAH may indicate that therapeutic concentrations were achieved in appropriate compartments, including the microvascular bed and cerebrospinal fluid, as a consequence of the traumatic SAH. Selfotel was also found to have poor brain penetration. Furthermore, in TBI, the frequent occurrence of low cerebral blood flow early after injury would reduce drug delivery to the brain even more.25 Without adequate crossing of the BBB, this competitive NMDA receptor antagonist would not be expected to interfere with the binding of endogenous glutamate to its receptor.
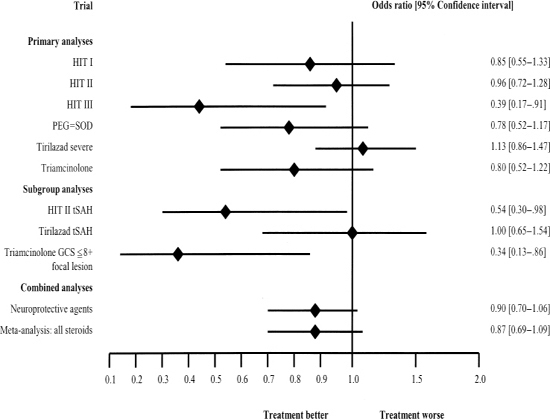
Figure 23-1 Summary of treatment results of several head injury trials. From Maas AI, Steyerberg EW, Murray GD, et al. Why have recent trials of neuroprotective agents in head injury failed to show convincing efficacy? A pragmatic analysis and theoretical considerations. Neurosurgery 1999;44:1286–1298.
Pearl




