Fig. 1
Schematic description of some of the experimental loci discussed in the text. SMA Supplementary motor area, PFC Prefrontal cortex, PMC Premotor cortex, SSC Somatosensory cortex. L-S corresponds to Libet’s stimulation of the somatosensory brain area. L-R1 is the recording of RP from SMA of Libet (and others). S-R1 and S-R2 correspond roughly to the brain areas, where Soon et al. detected nerve signals 10s before the awareness of a willful act. D-S1 and D-S2 correspond roughly to the areas stimulated by Desmurget et al. F-S is the stimulation to the SMA by Fried et al.
3 Analysis and Computer Simulations
Experimental results, such as those briefly summarized above have been taken as evidence that free will is an illusion, since a neural signal apparently associated with the conscious will precedes it by a substantial time period. However, in a complex system such as the human brain, constantly interacting with its environment and with extensive feedback loops, it is difficult, if not impossible to determine any causal chains. We are in a continuous perception-action cycle, where cause and effect cannot easily be discerned. The situation is complicated by the different levels of organization of our nervous systems, where there are not only loops between different parts of the brain, but also between different levels (micro, meso, macro). We may not be able to say with certainty whether neural events precede mental events, or vice versa, or whether they are simultaneous.
Careful analysis of the experimental results above actually shows that several alternative interpretations may be equally probable, including (unconscious/unreported) pre-planning. Libet’s experiments demonstrate a strong dependency on pre-planning, as discussed above. This means that the results in all experiments of this type, including those by Soon et al. [1], may be compromised by the presence of an unknown amount of pre-planning. In addition, when Soon et al. find a 60 % correlation between the next decision and the state of the PFC 10 s before the action, could that reflect a correlation between the memory of previous choices and the upcoming decision. It is conceivable that the next choice in the series could have a 60 % correlation with the accumulated history of the series, since humans are not very good at random number generation, which may be sought (unconsciously) by the subjects in these experiments.
Libet concluded there was a subjective backward referral, explaining the (false) sensation of a causative conscious will. Libet used, however, a threshold stimulus to the cortex. The electrical current was set so that it was just above the limit where it became noticeable. However, Pollen [20] showed that the 500 ms delay is an artifact of using threshold stimuli. Repeating Libet’s threshold stimuli experiments on the visual cortex of anesthetized cats, Pollen elucidated the mechanism for time-delay of such inputs and found that neural inhibitions delays the expression of threshold stimuli. Normal sensory stimuli are presumably well over threshold and enters awareness much faster. The bulk of Libet’s observations is thus neatly explained by Pollen, without revoking to the hypothesis of subjective backward referral.
Further, applying Bayesian network analysis to the Soon et al. [1] experiments, where multivariate probabilities are discussed, demonstrate that the alternative interpretation, that conscious will is causative, is also consistent with all experimental and anatomical facts.
In order to investigate the spatiotemporal relations between local and global events and processes in a cortical network, we have used computer simulations with cortical network models [21–24]. In particular, a spontaneous pulse in one part of the network may result in oscillatory (about 40 Hz) spatiotemporal patterns of activity in the entire network half a second, or more, later (see Fig. 2). This could be interpreted as an intentional, spontaneous, impulse in one part of the brain that gives rise to an extensive spatiotemporal activity pattern that would correspond to a conscious experience of the intentional impulse. In this particular case, the approximately 500 ms delay between the impulse and the oscillatory pattern could correspond to the time for a subjective experience to build up. Longer time delays could be modelled with larger and more complex network models (which will be published elsewhere).
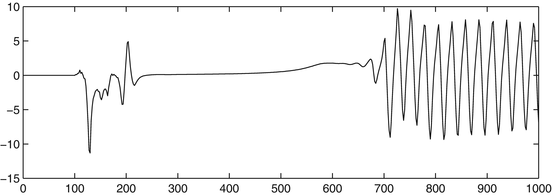

Fig. 2
Simulation results of a pulse in a cortical network node resulting in a global oscillatory dynamics of the entire network some 500 ms later. (The y-axis corresponds to EEG amplitude at an arbitrary scale, the x-axis denotes time in milliseconds)
4 Discussion and Conclusions
In this paper we have focused on a series of neurophysical experiments, which has been used in the debate as evidence for an epiphenomenal conscious will. Many of these experiments are largely based on the findings that some “signal”, e.g., the so-called readiness potential (RP), precedes a conscious will to perform a movement.
The general conclusion (e.g., [1, 25]), is that neuroscience finally has abolished free will, by showing that real decisions are made subconsciously up to 10 s before the illusory conscious act of will. A conservative interpretation of the same data is, however, that there is a weak correlation between the brain state long before the action and the experienced decision to act. This correlation could alternatively be understood as an effect of occasional unreported pre-planning, a correlation between the memory of the previous action and the next action, or a weak correlation between unconscious precursor processes and a causally connected conscious will.
With computer simulations, we have also demonstrated that a spontaneously initiated pulse in a single network node can result in a coherent global network activity much later. In reality, such an (intentional) impulse is not arising in a vacuum, but is embedded in a continuous flow of neural activity in a complex network of neurons and cortical subsystems, where the causal relationships are difficult, if not impossible to follow. Nevertheless, it is conceivable that the intentional impulse is part of a conscious experience, where the sense of a “self” acting needs time to emerge. Intention may indeed precede attention, in the perpetual action-perception cycle of consciousness exploring its environment [26].
In fact, when carefully examining the experimental procedures and results we can find no convincing evidence that conscious will is epiphenomenal. Similar conclusions have been made by others (e.g., [27–30]), including arguments from animal behavior and clinical studies of mental malfunction. Batthyany [27] points at the bias in interpretation of the experiments above, where the dominating philosophical preference suppresses alternative interpretations. The alternative hypothesis of a causative conscious will cannot be falsified or confirmed by the evidence either. In fact, it is not easy to design and perform experiments that could reveal the true nature of willful acts, especially not in an artificial environment with non-ecological tasks. One could argue that neither Libet nor Soon et al. test for free will at all, since the subjects are only asked to perform an “action” when there is an urge to move, and these movements can be said to be actions only in a very limited sense.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







