Relationship between plasma fluphenazine levels and estimated probability of improvement (gray line) and disabling side effects (black line) [20].
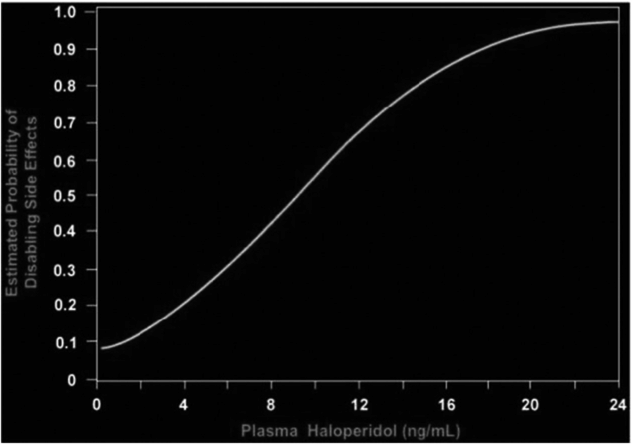
The relationship between plasma haloperidol and the estimated probability of disabling side effects [20].
It should be acknowledged that there are rare circumstances in which schizophrenia patients may exhibit no threshold for neurological adverse effects. The data in Table 22.2 are from a trifluoperazine titration study in which 10 male patients with schizophrenia were started on trifluoperazine 15 mg/d (equivalent to 6 mg haloperidol) without antiparkinsonian treatment, and advanced by 10 mg per week until they reached a predefined level of neurological adverse effects [14]. Subject 5 remained in the titration arm for over 36 weeks, achieving a daily trifluoperazine dose of 480 mg, yet had the lowest neurological side effect rating among the subjects in this study despite obvious evidence of significant drug exposure (weight gain). In such individuals, an antipsychotic trial may be terminated for futility (Principle 4b), although no definite guidelines exist. Clinical experience within the DSH system has yielded very few responders at fluphenazine levels significantly greater than 4.0 ng/ml, haloperidol levels above 30 ng/ml, and olanzapine levels above 200 ng/ml.
| Patient | Age (years) | Length of hospitalization (years) | Maximum daily trifluoperazine dose (mg) | Degree of psychiatric improvement* | Maximum neurological rating | Weight change (lbs) |
|---|---|---|---|---|---|---|
| 1 | 35 | 16 | 30 | – | 3.0 | –5 |
| 2 | 52 | 31 | 150 | – | 1.2 | 5 |
| 3 | 52 | 27 | 100 | 0 | 1.5 | –3 |
| 4 | 23 | 6 | 220 | + | 1.1 | 25 |
| 5 | 30 | 11 | 480 | + | 0.4 | 30 |
| 6 | 41 | 23 | 100 | ± | 1.1 | 10 |
| 7 | 51 | 14 | 20 | + | 3.1 | 10 |
| 8 | 57 | 16 | 30 | +++ | 2.0 | 12 |
| 9 | 42 | 11 | 40 | ± | 2.0 | 12 |
| 10 | 55 | 32 | 60 | + | 2.0 | 6 |
| Mean | 43.8 | 18.7 | 123 | 1.74 | 10.2 |
* Interpretation of psychiatric symptom change: – =no improvement with later worsening; 0 = no improvement; ± = mild improvement with later worsening; + = mild improvement; + + = moderate improvement; + + + = marked improvement.
The principles listed above do not cover details such as the rapidity of titration or duration for any dosage, as these parameters are often dictated by clinical acuity. Recent analyses of industry-sponsored schizophrenia trials do indicate that, with respect to symptom reduction, the absence of any treatment effect after 2 weeks predicts nonresponse at week 6, and should prompt reconsideration of the treatment approach [30]. In many instances, the response to the lack of improvement will include documentation of plasma levels and consideration of dose increases when tolerability is not yet an issue. In patients who have failed high doses of high-potency first-generation antipsychotics with supporting plasma level data, olanzapine is an option for those who cannot or will not take clozapine, bearing in mind its inferiority to clozapine [11].
Case 1: A High-Dose Responder: Plasma Level Guided High-Dose Antipsychotic Therapy
The patient is a 50-year-old African-American female with a diagnosis of schizophrenia, and multiple medical problems including type 2 diabetes mellitus (DM), who was admitted to a forensic psychiatric hospital following a conviction for manslaughter. Owing to ongoing assaultiveness and suicidality, she was started on clozapine with significant clinical response, but also the development of poorly controlled DM, necessitating the withdrawal of clozapine therapy. Trials of other atypical antipsychotics at high dosages proved ineffective in managing her psychotic symptoms and violence, so the decision was made to embark on a course of high-dose haloperidol therapy, given her prior tolerance for lower dosages. Over several months, the patient was titrated to a daily dose of 45 mg with a marked reduction in psychotic symptoms, requirements for PRN medications, and acts of aggression toward herself and others. Neither EPS nor akathisia were evident, and she was not maintained on routine antiparkinsonian medication. A new psychiatrist assumed the patient’s care and obtained a plasma haloperidol level of 29.5 ng/ml. Alarmed that this greatly exceeded the laboratory reference range (5–12 ng/ml), the haloperidol dose was decreased to 20 mg/d (plasma level 8.1 ng/ml). Over the next month, the patient deteriorated, started to refuse routine medication, and required numerous PRN medications for stability. The haloperidol dose was increased to 30 mg for several months, but the patient was frequently assaultive despite this dose increase and the addition of adjunctive lithium and lamotrigine. Another psychiatrist agreed to assume the patient’s care, but the patient continued to intermittently refuse oral haloperidol, primarily for delusional reasons. The patient was convinced to try fluphenazine in lieu of haloperidol, and titrated to 20mg/d with a trough plasma level of 0.7 ng/ml, but limited clinical improvement. To increase the plasma level and also to limit any confounding issues related to adherence, fluphenazine decanoate was started at 25mg every 2 weeks, oral fluphenazine was tapered off, and the depot dose increased over ensuing months to 100mg every 2 weeks. With moderate improvement on this regimen and the absence of EPS and akathisia, the psychiatrist chose to supplement fluphenazine decanoate with oral fluphenazine 15 mg with marked reduction in psychosis and complete resolution of aggressive acts. The plasma fluphenazine level on this combination was in the range 3.0–3.2 ng/ml.
Commentary
Although a plasma haloperidol level of 29.5 ng/ml would be intolerable for the vast majority of patients, this patient clearly demonstrated both a requirement of, and tolerability for, a very high level of D2 blockade, with significant clinical deterioration at lower antipsychotic levels. The latest treating psychiatrist correctly recognized the clinical situation and convinced the patient to try a similar medication to haloperidol, fluphenazine. The patient’s response to a second high potency first-generation antipsychotic at extremely high plasma levels is consistent with the prior clinical history. The lack of significant adverse effects also supports continued fluphenazine treatment with dosages required to maintain a plasma fluphenazine level ≥ 3.0 ng/ml.
Case 2: High-Dose Failure: The Value of Plasma Levels
The patient is a 29-year-old Latino with a diagnosis of schizophrenia, who was admitted to a forensic psychiatric hospital after being found incompetent to stand trial on numerous charges including assault with a deadly weapon, criminal threats, and arson of an inhabited structure. The court commitment included an involuntary medication order in the case of treatment refusal. The patient was admitted to the hospital from custody on a combination of oral olanzapine 40 mg at bedtime and divalproex 1000 mg twice per day, but extent of adherence was unknown, and he was grossly psychotic in the admission suite, requiring intramuscular medications to control agitation. The medication combination started in jail was continued with good adherence, as documented by trough plasma olanzapine levels of 73 ng/ml and 79 ng/ml and therapeutic serum valproate levels, but on hospital day 12, the patient engaged in an unprovoked assault on a peer with serious injury, and required seclusion and restraints.
Given the severity of the assault, the ongoing significant level of psychotic symptoms, and records from a prior hospitalization that this patient tolerated oral haloperidol doses of 20 mg/d or more, a decision was made to load the patient on haloperidol decanoate while continuing olanzapine and divalproex at prior dosages. The most aggressive depot haloperidol load permissible in DSH facilities is 300mg weekly for three doses; this regimen is designed to mimic plasma levels achievable with 30 mg/d of oral haloperidol [28]. This dosing equivalency is an extrapolation of prior loading studies demonstrating that, after three weekly injections of haloperidol decanoate 100 mg, mean plasma haloperidol concentrations from the depot were comparable to 10 mg/d of oral haloperidol (7.95 ± 4.94 ng/ml vs. 7.79 ± 4.79 ng/ml) [31]. The three loading injections of 300 mg were administered in weekly intervals without evidence of adverse effects. Of note, a plasma haloperidol level obtained 1 week after the last loading injection (i.e., at TMax) was 6.4 ng/ml. An assault occurred two days after the third loading injection, but the patient was subsequently free from assaultive behavior and continued to show no evidence of EPS or akathisia. However, his psychotic symptoms appeared to worsen slightly in the week prior to the scheduled maintenance depot dose, so haloperidol decanoate 300 mg was administered 22 days after the last loading injection. A haloperidol level obtained 4 days later was 2.8 ng/ml. The following week, this patient was again involved in a serious unprovoked assault that necessitated seclusion and restraints. A psychopharmacology consultation was obtained to assist with management. Based on the unexpectedly low plasma haloperidol levels obtained despite aggressive loading, it was concluded that this patient’s inadequate response to haloperidol was a kinetic failure, but one that might not be easily overcome without the administration of extremely high weekly or biweekly injections of haloperidol decanoate – doses that would be unlikely to be continued outside of the forensic setting due to unfamiliarity with the rationale for a high-dose regimen in this patient. As this patient manifested expected plasma levels from oral olanzapine, a high-dose strategy for this medication was recommended, noting that doses of 60–80mg/d might be required to achieve plasma levels in the 100–150 ng/ml range, levels associated with 80%–85% D2 occupancy.
Commentary
In the absence of plasma haloperidol levels, one might conclude that this patient was one of the unusual individuals who does not experience EPS or akathisia, and is likely a pharmacodynamic failure of haloperidol treatment. As noted above, an expected haloperidol level after three weekly loading doses of 100mg haloperidol decanoate is approximately 7 ng/ml [31]. The fact that this patient’s haloperidol level 1 week after his third 300mg dose was 6.4 ng/ml was critical to the conclusion that this patient was a kinetic failure, and most likely was an ultrarapid metabolizer for medications such as haloperidol that utilize cytochrome P450 (CYP) 2D6 [32], but not for those metabolized via CYP 1A2 (e.g., olanzapine) based on plasma olanzapine levels.
Conclusions
For treatment-resistant and violent patients with schizophrenia, there are limited therapeutic options, with clozapine and high antipsychotic plasma levels having the greatest evidence base. High plasma level antipsychotic therapy thus remains a viable strategy when employed in a rational manner as enumerated by the principles above. The appropriate use of plasma antipsychotic levels is central to the management of high-dose antipsychotic regimens, but must be complemented by documentation of changes in target symptoms and tolerability. Ongoing tolerability without efficacy represents a failure to achieve one of two firm clinical endpoints, and provides a sound basis for continued dose advancement despite what might be considered supratherapeutic plasma levels for average patients with schizophrenia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







