Subtraction lesions maps for the mPFC group (red), lPFC group (green), and non-PFC group (blue). For each group, the subtraction lesion map shows those brain areas that were more lesioned in 1 group compared to the other groups. Note that each subject was only included in 1 group.
Genetic analysis
From a published SNP array [24], we selected the following functional SNPs: DRD1 rs686 (A-to-G), DRD2 rs4648317 (C-to-T), and COMT Val158Met rs4680 (G-to-A). Rs686 is a functional SNP located in the promoter region of the DRD1 gene; its A allele is linked with increased transcriptional activity compared to the G allele [25]. Rs4274224 is located in the first intronic region of the DRD2 gene; the minor allele has been linked with reduced D2 expression in healthy controls compared to the major allele [26]. Moreover, this SNP has been shown to impact behavioral inhibition [27]. COMT Val158Met rs4680 is a widely studied SNP in neuropsychiatry, and the Val allele is thought to be linked with a reduced efficiency in the degradation of dopamine [16]. Last, we decided to control for possible differences among subjects for the monoamine oxidase A (MAO-A) genotype, one of the genes more commonly linked to pathological aggression [2,28]. While the main role of the MAO-A is serotonin metabolism rather than dopamine, we decided to include it as a confounding factor since the common variable number tandem repeat (VNTR) polymorphism is known to impact aggression levels in the general population [28]. VNTR MAO-A polymorphisms are thought to modulate MAO-A activity with the 3.5 and 4 repeats linked with MAO-A high-activity and 2, 3, and 5 repeats linked with low MAO-A activity. Genotyping for those SNPs was performed as described elsewhere [2,18,24].
Statistical analysis
Statistical threshold was set at 0.05 (2-tailed) for all first-level analyses. For each of our target SNPs (DRD1 rs686, DRD2 rs4648317, and COMT Val158Met rs4680), a mixed 2 × 4 analysis of covariance (ANCOVA) on NPI-a (and NPI-t as a control measure) was performed with Genotype (major allele/–, minor allele/minor allele) and Group (mPFC, lPFC, non-PFC, control) as between-subjects factors and the other target genes as covariates. Based on our DRD1 results (see below), we also performed a 2 × 4 ANCOVA on NPI-a scores (and NPI-t scores) with DRD1 Genotype and Group as between-subjects factors and DRD2, COMT, and MAO VNTR genotype as covariates. Follow-up independent t-tests (Bonferroni corrected for multiple comparisons) were performed for the rs686 genotypes within each lesion group.
Results
Group characteristics
All four groups did not present with significant differences in pre-injury intelligence (F(3,165)=1.2, p = 0.31), early life negative experiences (F(3,165)=0.7, p=0.55), education level (F(3,165)=0.8, p=0.50), and age (F(3,165) = 0.4, p = 0.75), and the lesion groups were matched on percentage of brain tissue loss due to pTBI (F(2,137) = 0.9, p = 0.41) (Table 10.1). Lesion subtraction maps are reported in Figure 10.1. Frequency distributions for the genotyping results were as follows: DRD1: 97 A/– vs. 73 G/G subjects; DRD2: 117 C/– vs. C/– subjects vs. 53 T/T; COMT: 107 Val/– vs. 63 Met/Met subjects; MAO-A: 59 low-activity vs. 111 high-activity subjects.
| DRD1 rs686 group | NPI-a | NPI-t | Pre-injury IQ | Age | Education | % of brain volume loss | ETI |
|---|---|---|---|---|---|---|---|
| mPFC lesion group | |||||||
| A/– (n = 22) | 2.70 ± 0.2 | 5.6 ± 0.8 | 59.5 ± 4.6 | 57.5 ± 0.4 | 14.6 ± 0.4 | 3.2 ± 0.4 | 5.6 ± 0.4 |
| G/G (n = 34) | 0.52 ± 0.2 | 5.1 ± 0.7 | 58.0 ± 4.2 | 58.8 ± 0.4 | 14.3 ± 0.3 | 3.4 ± 0.4 | 5.9 ± 0.3 |
| lPFC lesion group | |||||||
| A/– (n = 20) | 0.46 ± 0.2 | 5.2 ± 0.7 | 58.5 ± 5.6 | 58.8 ± 0.3 | 14.3 ± 0.4 | 4.0 ± 0.6 | 5.5 ± 0.5 |
| G/G (n = 31) | 2.40 ± 0.3 | 5.6 ± 0.9 | 59.6 ± 4.1 | 58.2 ± 0.5 | 15.0 ± 0.5 | 3.9 ± 0.7 | 5.8 ± 0.3 |
| Non-PFC lesion group | |||||||
| A/– (n = 14) | 1.22 ± 0.3 | 5.6 ± 0.8 | 63.7 ± 5.8 | 58.2 ± 0.3 | 14.8 ± 0.4 | 3.0 ± 0.3 | 6.0 ± 0.5 |
| G/G (n = 20) | 1.44 ± 0.2 | 5.4 ± 0.8 | 62.2 ± 6.0 | 58.6 ± 0.5 | 14.9 ± 0.5 | 3.2 ± 0.4 | 5.9 ± 0.6 |
| Control group | |||||||
| A/– (n = 10) | 0.93 ± 0.4 | 5.4 ± 0.6 | 60.2 ± 8.6 | 58.2 ± 0.6 | 15.4 ± 0.8 | N/A | 6.0 ± 0.4 |
| G/G (n = 19) | 1.12 ± 0.2 | 4.9 ± 0.5 | 61.2 ± 9.4 | 59.1 ± 0.3 | 15.5 ± 0.6 | N/A | 5.9 ± 0.5 |
Note: NPI-a: Neuropsychiatric Inventory Aggression sub-score; NPI-t: Neuropsychiatric Inventory Total score; ETI: Early Trauma Inventory Score.
DRD1
The ANCOVA on NPI-a revealed a significant interaction effect for DRD1 Genotype × Group (F(3,159) = 9.5, p = 0.001), but no significant main effects were found for DRD1 Genotype (F(1,159) = 0.4, p = 0.58) and Group (F(3,159) = 1.2, p = 0.35) and no covariate effect was found for COMT (F(1,159) = 0.45, p = 0.55), DRD2 (F(1,159) = 0.60, p = 0.4) or MAO-A (F(1,159) = 0.35, p = 0.35). Planned follow-up analyses showed that DRD1 A/– carriers had higher NPI-a scores than G/G carriers in the mPFC group (t = 2.99, p = 0.004; Cohen’s d = 1.9), whereas DRD1 A/– carriers had lower NPI-a scores than G/G carriers in the lPFC group (t = 3.82, p = 0.002; Cohen’s d = 1.89) (Figure 10.2). No significant differences were found between genotypes in the non-PFC (t = 0.92, p = 0.36) and control (t = 0.72, p = 0.48) groups.
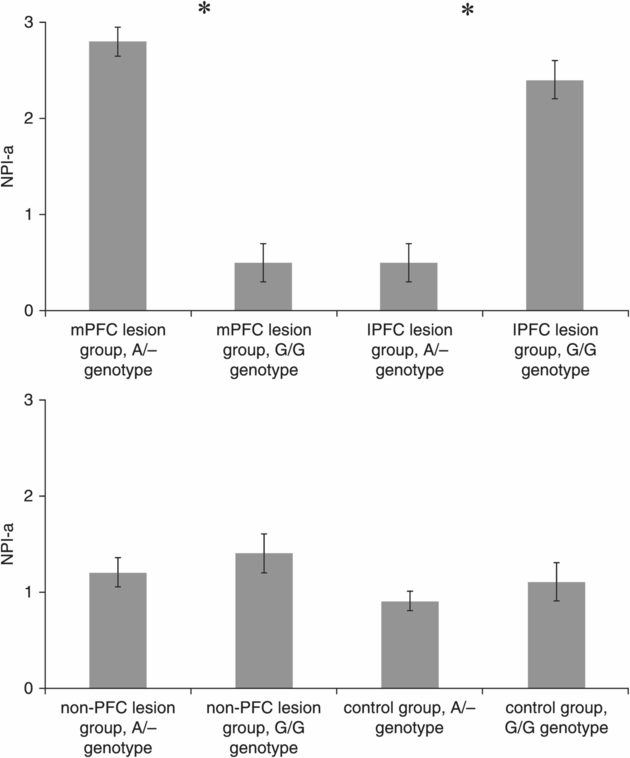
NPI-a scores (mean ± s.e.m.) for the lesion groups divided according to the functional SNP DRD1 rs686. *Indicates a significant difference between the two genotype groups at p < 0.05 (Bonferroni correction).
The ANCOVA on NPI-t revealed no significant main effects (DRD1 Genotype: F(1,159) = 1.5, p = 0.25; Group: F(3,159) = 0.85, p = 0.38); interaction effect (F(3,159) = 1.1, p = 0.37); or COMT, DRD2, and MAO-A covariate effects.
DRD2
The DRD2 × Group ANCOVA performed on NPI-a scores revealed no significant main effects (DRD2 genotype: F(1,159)=1.7, p=0.52; Group: F(3,159)=0.5, p=0.80); interaction effect (F(3,159)=0.15, p=0.92); or DRD1, COMT, and MAO-A covariate effects.
COMT
The COMT Genotype × Group ANCOVA performed on NPI-a scores revealed no significant main effects (COMT genotype: F(1,159)=0.7, p=0.40; Group: F(3,159)=0.2, p=0.89); interaction effect (F(3,159)=0.15, p=0.92); or DRD1, DRD2, and MAO-A covariate effects.
Discussion
In this study, we studied the relationship between TBI-related aggression and the dopaminergic system and its modulation by lesion location. Our results revealed a significant interaction between aggressive behavior and the DRD1 rs686 SNP depending upon PFC lesion location. We showed that carriers of the major and more transcriptionally active allele of DRD1 were more aggressive compared to the minor allele homozygotes in the mPFC group, while conversely, DRD1 major allele carriers were less aggressive than minor allele homozygotes in the lPFC group.
Moreover, we did not observe any significant interactions between lesion location and genotype for DRD2 or COMT functional SNPs, thus suggesting a possible specificity of this effect to DRD1 receptors. Last, no genotype effects were observed in the non-PFC and control groups or when taking into account the global index of psychopathology.
We propose that our observation of high aggression levels in two specific subsets of pTBI patients – (1) subjects with mPFC lesions and more expressed DRD1 receptors and (2) subjects with lPFC lesions and less expressed DRD1 receptors – can be explained by analogy to the known relationship between impaired cognitive performance and excessively high or low levels of dopamine (i.e., the observed “U-curve” relationship between cognitive performance and dopaminergic tone) [29].
While the “U-shaped curve” relationship between function and dopamine levels was first observed at the neural level, recent years have seen its generalization to the behavioral, whole-organism setting. In rats, for example, both D1 agonists and antagonists have been shown to impair working memory performance in a dose-dependent function, leading to a U-shaped curve-like relationship (i.e., rats presented an equally pathological performance both during excessive inhibition and excessive stimulation of the D1 receptors) [30]. Moreover, in line with the proposed U-shaped curve relationship between dopamine tone and performance, a DRD2 agonist, cabergoline, increased neural reward responses during a feedback-based reversal learning fMRI task in healthy subjects with low DRD2 receptor density due to the A1+ Taq1A SNP, while it reduced reward responses in those subjects with higher DRD2 receptor density due to the A– Taq1A SNP [17]. Last, a dopamine agonist, bromocriptine, has been shown to increase cognitive performance in healthy subjects with lower baseline dopamine synthesis – as quantified with fluoro-L-m-tyrosine PET scans, but showed an opposite effect (i.e., a performance reduction) in subjects with higher baseline dopaminergic tone [31].
Our findings suggest that subjects with lPFC lesions and less expressed DRD1 represent the other extreme of the proposed “U-shaped curve” relationship between dopaminergic tone and aggression (i.e., association of high levels of aggression with reduced D1 signaling). This proposal is in line with the observed reduction of deep brain dopaminergic activity in lPFC virtual-lesion studies based on theta-burst TMS13 and an increased striatal dopaminergic tone after lPFC activation [14].
Thus, the combination of reduced dopaminergic activity with low DRD1 expression could lead to low D1 tone in different subcortical areas linked with aggression, such as the nucleus accumbens (NA). According to pharmaco-fMRI studies, low D1 activity is linked with blunted NA responses to environmental stimuli [32]. A similar blunted NA response to environmental stimuli has been shown in subjects with ADHD, and it has been correlated with the extent of externalizing symptoms, which include aggressive conduct [33]. Interestingly, pro-dopaminergic stimulants are widely used to control externalizing aggressive behaviors in ADHD [9], as well as in some experimental models of aggression [34]. Last, a relationship between NA neuro-degeneration and disinhibited behavior has been shown in FTD in which pro-dopaminergic stimulants are used to treat aggression [35].
Our findings also indicate that subjects with mPFC lesions and more expressed DRD1 represent the “high dopamine” end of the proposed “U-shaped curve.” The mPFC territories are richly interconnected with the ventrotegmental area (the origin of the mesolimbic dopaminergic pathway to the NA), which suggests a modulatory effect of mPFC on the dopaminergic system [36], as shown in animal lesion models in which the structural lesion of medial PFC territories was linked with an increase in NA dopaminergic activity [15]. These observations are in line with the observed activation by mPFC projections of inhibitory GABA-ergic inter-neurons in the dopaminergic mesolimbic pathway [36], as well as with the reported inverse correlation between mPFC and NA activity during immediate reward evaluation in impulsive subjects [37]. Given the relationship between D1 signaling and NA activity, the presence of mPFC lesions in subjects with highly expressed DRD1 could lead to an excessively active NA, especially in behaviorally relevant impulsive decision-making settings, and possibly to heightened aggressive behaviors [38].
We propose that our finding of high aggression levels in pTBI subjects with mPFC lesions and more expressed DRD1 and in subjects with lPFC lesions and less expressed DRD1 supports a U-shaped curve modulation of the function of the mesolimbic dopaminergic system. Coincidentally, in a recent meta-analysis of striatal activation during reward anticipation tasks, a similar U-shaped curve paradigm has been proposed to link the reduced reward responsiveness of mesolimbic striatal structures observed in subjects with extremely high or extremely low impulsivity observed in ADHD and healthy control group [39]. Our findings have a potential translational application in the pharmacological treatment of behavioral disturbances in pTBI patients [40]. Lesion location could represent a low-cost, easy-to-use, para-clinical marker to help, among other factors, in the development of individualized treatment protocols.
Indeed, our findings seem to suggest that in subjects with isolated lPFC lesions D1-agonists could represent the treatment of choice for aggressive behaviors, while the compounds in this class should be avoided in subjects with isolated mPFC lesions.
Albeit indirectly, moreover, our data also advise prudence in the use of D1-active drugs in subjects with mixed mPFC/lPFC, even if our study did not directly investigate this patient group. However, while this proof-of-concept study suggests the importance of a personalized approach to aggression treatment in pTBI, future studies are needed to explore its relevance in day-to-day clinical care.
Interestingly (but unexpectedly), given the widespread use of D2-antagonists to treat behavioral disturbances in pTBI [41], we did not find any effect of the DRD2 SNP on aggression in our target populations. We argue that this observation, while it needs to be interpreted with caution, is in line with the growing evidence of the difficulties in generalizing findings from general psychiatry to neuropsychiatry. Indeed, different studies showed that chronic reduction of D2 tone using DRD2 inhibitors after TBIs increases the risk of stable cognitive deficits [42,43], which, given the relationship between cognitive deficits and behavioral disturbances in pTBI, could counter the potentially positive effect of D2 inhibition on aggressive behaviors in this population.
One of the key aspects of our study is the composition of our patient group. Our experimental group is highly homogeneous regarding pTBI (all subjects suffered combat-related pTBI during their service in Vietnam), their demographic characteristics (all subjects suffered pTBI during their early adulthood), and their pre-injury cognitive levels. Moreover, all subjects were matched for their early negative experience burden and their exposure to aggressive environments (i.e., all of them were exposed to infantry warfare and suffered a major injury). While this homogeneity allowed us to control for possible confounding factors (e.g., pre-injury characteristics, TBI dynamics, and exposure to significant aggressive behaviors), it also represents the main limitation of this study, which prompts the need to also evaluate our findings in non-military heterogeneous populations. Another limitation of this study is the lack of anatomical information on pTBI-related white matter damage, since retained metal fragments in the brain preclude high resolution structural MRI studies.
Moreover, in this study we focused on DRD1 and DRD2, as they represent the prototypical members of the two families of dopamine receptors (i.e., the DRD1-like and the DRD2-like families) [15]. Future studies, however, are warranted to explore the role of other dopaminergic receptors in behavioral disturbances in pTBI, especially taking into account the differences in the anatomical localization of the different receptors. DRD4, for example, seems to be of particular interest, as it is widely expressed in the PFC [44] and been associated with interindividual differences in externalizing behaviors [45], as well as with resilience after negative life experiences [46].
The longitudinal aspect of our study allowed us to evaluate the behavioral consequences of pTBI across the patients’ lifetime, which are a major determinant of quality of life levels both for our patients and their caregivers. Furthermore, the length of our follow-up suggests caution in the interpretation of our data, especially regarding their generalizability to the acute and sub-acute settings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






