Antipsychotic Depot Injections
Administration
Long-acting antipsychotic depot injections may be administered to adults by deep intramuscular injection. They should not be given to children.
Advantages and disadvantages
This form of administration leads to excellent compliance with the medication, as there is slow and sustained absorption of the antipsychotic drug from the depot. Disadvantages of using depot injections include:
The injections may cause false results from investigations such as the creatine kinase level.
Extrapyramidal symptoms may occur more often with depot preparations of first-generation antipsychotics than with the corresponding oral preparations. However, depot preparations of second-generation antipsychotics (such as paliperidone and olanzapine embonate), with which such symptoms may be less common, are available.
They should be used with caution if a patient is taking anticoagulant medication as this may increase the bleeding time.
General side-effects can include pain, erythema, swelling, nodules, and damage to anatomical structures such as nerves.
Site of injection
The injection of a small volume (maximum 2mL), such as a test dose, using a very small needle can be given into the deltoid muscle in the upper arm. In general, however, with the exception of paliperidone and risperidone (for which the deltoid muscle can be chosen), deep intramuscular injections are given into muscles of the lower limb. There are 2 common choices:
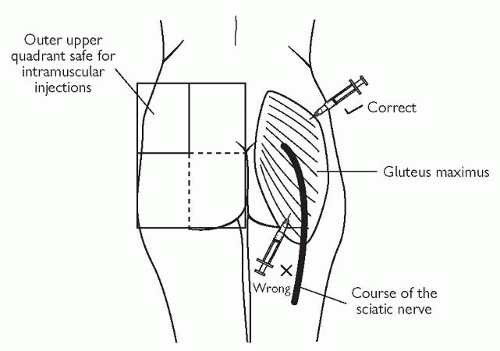
The buttock. This has the major disadvantage of the possibility of damage to the sciatic nerve. If the buttock is to be used, then ideally the whole buttock should be exposed, the upper and outer quadrant of the buttock thereby identified, and the injection given into this upper outer quadrant (see Fig. 4.1). With a relatively long needle such an injection is more likely to enter the gluteus medius rather than the gluteus maximus. With too short a needle the depot injection is more likely to be deposited in fat rather than muscle. In general, manufacturers of depot antipsychotic medication recommend the gluteal muscles.
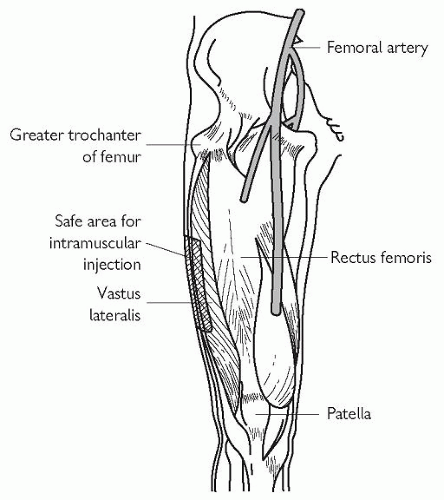
The lateral thigh. The injection is deposited into the vastus lateralis. In general this is safer than trying to inject into the gluteus medius or gluteus maximus. The vastus lateralis is part of the quadriceps femoris; a suitable part of the muscle into which to inject can be found by placing a hand below the greater trochanter of the femur and another hand above the lateral condyle of the femur—the mid-lateral thigh region in between is a suitable area, as shown in Fig. 4.2. (The greater trochanter lies inferior to the middle of the iliac crest, while the lateral femoral condyle is easily felt at the knee, particularly when the knee is flexed.)
When choosing a site for intramuscular injection, the following sites should not be chosen:
birthmark
inflammation present
irritation present
mole
oedema present
scar tissue.
Z-track technique
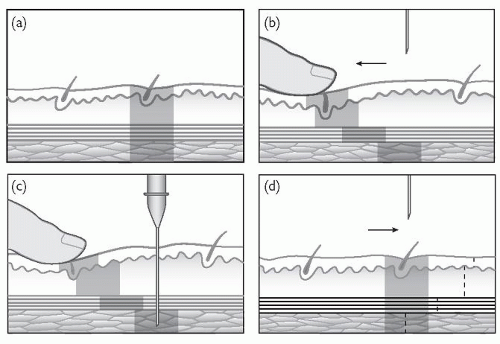
Normally, the skin in section is as in Fig. 4.3a. The skin is displaced with a finger as in Fig. 4.3b. The needle is then inserted as in Fig. 4.3c, and the depot injected. The needle is then withdrawn. Finally, your finger is removed and the skin returns to its original position as shown in Fig. 4.3d, thereby breaking the needle track and trapping the depot in the muscle without allowing for a ready route of exit of the drug into the subcutaneous tissue or the surface of the skin.
Dosage guidelines
The recommendation of the British National Formulary is:
‘Individual responses to neuroleptic drugs are very variable and to achieve optimum effect, dosage and dosage interval must be titrated according to the patient’s response.’
As mentioned in Chapter 3, The Royal College of Psychiatrists have issued the following advice on doses exceeding the British National Formulary upper limits (which constitutes unlicensed use).
Consider alternative approaches including adjuvant therapy and newer or second-generation antipsychotics such as clozapine.
Bear in mind risk factors, including obesity; particular caution is indicated in older patients, especially those over 70.
Consider potential for drug interactions.
Carry out ECG to exclude untoward abnormalities such as prolonged QT interval; repeat ECG periodically and reduce dose if prolonged QT interval or other adverse abnormality develops.
Increase dose slowly and not more often than once weekly.
Carry out regular pulse, blood pressure, and temperature checks; ensure that patient maintains adequate fluid intake.
Consider high-dose therapy to be for limited period and review regularly; abandon if no improvement after 3 months (return to standard dosage).
In addition, the British National Formulary offers the following advice:
‘Important: When prescribing an antipsychotic for administration on an emergency basis, the intramuscular dose should be lower than the corresponding oral dose (owing to absence of first-pass effect), particularly if the patient is very active (increased blood flow to muscle considerably increases the rate of absorption). The prescription should specify the dose for each route and should not imply that the same dose can be given by mouth or by intramuscular injection. The dose of antipsychotic for emergency use should be reviewed at least daily.’
Caution must be exercised in cases of:
hepatic impairment
renal impairment.
In such cases, if prescription of an antipsychotic depot is being considered, then reference should be made to the corresponding manufacturer’s product literature, and the manufacturer’s medical information department may need to be contacted for advice.
In general, these medications are contraindicated in pregnancy.
Equivalent doses
Equivalent doses for a selection of intramuscular depot antipsychotic drugs are given in the British National Formulary and reproduced in Table 4.1. Note that these equivalent doses should not be extrapolated beyond the maximum dose for each antipsychotic drug.
Table 4.1 Equivalent doses of depot antipsychotics. Reproduced with kind permission of the Joint Formulary Committee. British National Formulary, 51st edn (2006). British Medical Association and Royal Pharmaceutical Society of Great Britain, London. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree