CHAPTER 232 Electrodiagnostic Evaluation of Peripheral Nerves
Electromyography and Nerve Conduction Studies
Electromyography (EMG) and nerve conduction studies (NCSs), also referred to as electrodiagnostic studies, are used to test the function of the nervous system.1,2 They are generally used to test the integrity of the peripheral nervous system (PNS) but can also be used to evaluate movement disorders, such as cervical dystonia (torticollis). EMG and NCSs are best used as an extension of the neurological examination to help localize and define a lesion. They can help determine whether weakness or numbness is due to a lesion in the central nervous system (CNS) or PNS. Once a lesion is determined to be in the PNS, EMG and NCSs can localize the lesion to the anterior horn cell, nerve root, dorsal root ganglion, plexus, nerve, neuromuscular junction, or muscle. In addition, the degree of involvement of the sensory and motor nerves can be determined. Lesions can also be localized to the cell body, axon, or myelin. The pattern of abnormalities on EMG and NCSs is used to provide a definitive diagnosis. These electrodiagnostic studies can also be used to determine the duration, severity, and prognosis of a lesion. Finally, they can provide an objective measure of improvement or worsening, which is often useful when determining response to treatment.
Fundamentals of Electrodiagnostic Testing
EMG and NCSs use different means of measuring action potentials of nerve axons or muscle fibers. The physiology of an action potential is discussed elsewhere.1 Measurement of action potentials involves placing two recording electrodes along a nerve axon or muscle fiber (Fig. 232-1). The difference in electrical potential between the two electrodes is amplified through a differential amplifier and plotted on a monitor for analysis. Because the recording electrodes are close to each other (usually within a few centimeters), in the absence of an action potential, there is no significant difference in electrical potential between them. As an action potential approaches one of the electrodes, this electrode measures an electrical potential that is not measured by the other electrode. A triphasic wave is recorded as the action potential passes under the first electrode. Most recordings during EMG and NCSs involve the summation of a number of action potentials from nerve or muscle fibers. For example, a sensory NCS involves recording the summation of individual action potentials from all the hundreds or thousands of sensory axons of a particular nerve.
Sensory Nerve Action Potential
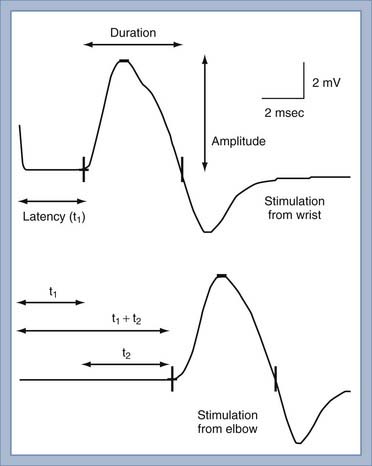
NCS of the sensory nerves generates a recording referred to as a sensory nerve action potential (SNAP). In this study, the sensory nerve is stimulated with sufficient electrical current that all the large-diameter sensory axons are simultaneously depolarized (Fig. 232-2). This stimulation is referred to as supramaximal stimulation because a higher electrical current than the minimum required for stimulation of all the axons is used to ensure that all the axons are depolarized. Action potentials of the depolarized axons immediately travel away from the site of stimulation at various velocities, depending on a number of factors. For instance, conduction velocity increases with larger axon diameter, increased myelination, and higher nerve temperature. The action potentials travel along the axons and are recorded by the recording electrodes over the nerve (Fig. 232-2). Each individual action potential generates a triphasic recording (see Fig. 232-1). A typical sensory nerve contains up to several hundred sensory axons, and an equal number of action potentials is recorded from the nerve. The SNAP is the sum of the individual action potentials recorded from each sensory axon. Under normal conditions, the action potentials of large-diameter sensory axons travel at similar velocities and thus pass under the recording electrode nearly simultaneously. The sum of these action potentials results in the SNAP (Fig. 232-3). The action potentials of small-diameter myelinated and unmyelinated axons travel at slower and more variable velocities. Thus, these action potentials pass under the recording electrode at variable times and do not summate sufficiently to generate enough amplitude to be a visible waveform. The amplitude of the SNAP is calculated from the baseline, or first positive peak, to the negative peak (while keeping in mind that negative is upward) and is a reflection of the number of normal large-diameter myelinated sensory axons. Conduction velocity is calculated by dividing the distance between the sites of stimulation and recording by the time that the first action potentials reach the recording electrodes, which is represented as the beginning of the upward slope from the baseline or first positive peak. Under normal circumstances, a longer distance between the stimulation and recording sites results in less synchronized action potentials from the variety of slow- and fast-conducting axons. This phenomenon is referred to as temporal dispersion and results in a decreased SNAP amplitude and increased duration when recording over large distances (Fig. 232-3).
In the presence of nerve injury or disease, there is often a change in SNAP conduction velocity or amplitude. Changes in the SNAP depend on the site and mechanism of the lesion, but all such lesions cause numbness, paresthesias, and other sensory symptoms. Any lesion at or distal to the dorsal root ganglion that causes wallerian degeneration results in fewer sensory axons, fewer action potentials recorded by the electrodes, and a decreased SNAP amplitude (Fig. 232-4A). Conduction velocity is normal or nearly normal because the remaining axons are myelinated and function normally. With marked axonal loss, conduction velocity may be slightly decreased because of the loss of faster conducting axons. Conduction velocity can be decreased to 80% of the lower limit of normal for mild axonal loss and to 70% of normal when SNAP amplitude is less than 50% of the lower limit of normal.
Demyelination distally between the sites of nerve stimulation and recording leads to slowing of the individual action potentials and thus more markedly reduced conduction velocity or increased latency (time from stimulation to the initial waveform) of the SNAP (Fig. 232-4B). Typical clinical manifestations include carpal tunnel syndrome with demyelination of the median sensory nerve at the carpal tunnel or a demyelinating polyneuropathy such as Guillain-Barré syndrome. Because acquired demyelinating lesions frequently result in varying degrees of slowing of the individual axons, there is often increased temporal dispersion. If the demyelination is severe enough, the action potential may be unable to continue propagating down the axon across the site of demyelination to the recording electrode, thereby resulting in conduction block. Conduction block, which is almost always caused by demyelination, can result in a low-amplitude or absent SNAP. In most cases of conduction block there is concomitant conduction slowing because some axons are demyelinated to the point of conduction block but others are only partially demyelinated, which causes conduction slowing. Thus, a low-amplitude SNAP with conduction slowing suggests a demyelinating lesion, whereas a low-amplitude SNAP without conduction slowing suggests a lesion causing axonal degeneration without primary demyelination.
A demyelinating lesion proximal to the point of nerve stimulation and recording leaves the distal nerve intact. A lesion causing axonal loss proximal to the dorsal root ganglion, such as at the root, results in wallerian degeneration of the proximal axon. However, the sensory axons distal to the dorsal root ganglion maintain their continuity to the cell body and thus remain normal. The SNAP remains normal under these circumstances (Fig. 232-4C), even in the presence of anesthetic sensations. A normal SNAP in a patient with numbness suggests that the lesion is proximal to the dorsal root ganglion (root or CNS) or is a proximal demyelinating lesion.
Compound Muscle Action Potential
NCS of the motor nerves generates a recording referred to as the compound muscle action potential (CMAP). Unlike the situation with a sensory NCS, the CMAP is recorded from the muscle and not the motor nerve. Similar to a sensory NCS, the motor nerve is stimulated with supramaximal stimulation such that all the motor axons are simultaneously depolarized. Action potentials from the depolarized axons immediately travel away from the site of stimulation at nearly identical velocities. The action potentials travel along the axons to the neuromuscular junction. Each motor axon innervates up to several hundred muscle fibers. Because the typical motor nerve contains up to a few hundred motor axons, the amplitude of the CMAP—a summation of action potentials from muscle fibers—is 100 to 1000 times the magnitude of the SNAP. When compared with sensory nerves, the motor axons of motor nerves are much more similar in diameter and degree of myelination, thereby resulting in more similar individual axonal conduction velocities and very little temporal dispersion (Fig. 232-5). Moreover, the duration of motor unit action potentials (MUAPs) is long enough that the degree of overlap is affected relatively little by temporal dispersion.
The conduction velocity of the distal motor nerve segment cannot be calculated for the CMAP as it can for the SNAP because the time from nerve stimulation to recording of muscle fiber action potentials includes the release of acetylcholine. Acetylcholine must diffuse across the neuromuscular junction and bind to the acetylcholine receptor before the muscle fiber action potential can be recorded. This process takes about 0.5 to 1 msec. Instead of calculating conduction velocity from a point of stimulation to the muscle, the time from stimulation at a distal point of the nerve to the onset of the CMAP—referred to as the distal motor latency—is compared with that in normal controls to determine whether distal conduction slowing is present. The conduction velocity of the proximal portion of the motor nerve can be calculated as described in Figure 232-5. The three most important aspects of the CMAP are the amplitude, conduction velocity, and distal motor latency.
In the presence of injury or disease of the motor nerve or muscle, there is often a change in CMAP conduction velocity, amplitude, or distal motor latency. Changes in CMAP depend on the site and mechanism of the lesion, but all such lesions cause weakness and possibly muscle atrophy in the case of denervation. The more common scenarios are presented in Figure 232-6. For the sake of illustration, consider a motor nerve consisting of two motor axons, each of which innervates two muscle fibers. Under conditions of normal PNS functioning, supramaximal stimulation of the motor nerve distally and proximally leads to action potentials in both motor axons and all four muscle fibers. The CMAP recordings consist of the sum of the muscle action potentials from all four muscle fibers (Fig. 232-6A).
When there is motor axonal degeneration from distal dying-back axonopathy, wallerian degeneration from a proximal lesion, or loss of motoneurons, only one of the two motor axons is present and can transmit an action potential to its two innervated muscle fibers (Fig. 232-6B). The other two muscle fibers cannot be stimulated, which leads to decreased CMAP amplitudes from both the distal and proximal stimulation sites.
In this model, conduction block between the distal and proximal sites of stimulation results in a characteristic CMAP abnormality (Fig. 232-6C). A typical demyelinating lesion, such as from entrapment neuropathy, may result in conduction block of one motor axon and conduction slowing of the other. Distal to the site of demyelination, the motor axons and myelin are normal. Thus, stimulation of the motor nerve distally results in activation of both motor axons and all four muscle fibers, which yields a normal CMAP amplitude and distal motor latency. Stimulation of the motor nerve proximal to the site of demyelination leads to action potentials in both motor axons. However, because one of the action potentials is blocked at the site of demyelination, it is unable to proceed with activation of the corresponding muscle fibers. The other action potential is slowed but can still activate two of the muscle fibers. Hence, the CMAP from the proximal point of stimulation is the sum of two muscle fiber action potentials; its amplitude and area are smaller than normal, and it is delayed because of conduction slowing. Therefore a scenario in which CMAP amplitude and area at the proximal point of stimulation are decreased in comparison to the distal site is indicative of a conduction block between the two points of stimulation. If there is a decrease in CMAP amplitude but area is maintained, temporal dispersion is suggested. Unlike sensory nerves, there is normally very little temporal dispersion of motor nerves, and its presence indicates an area of demyelination.
Late Responses
SNAP and CMAP studies are best at evaluating distal nerves. Stimulation and recording from nerves at proximal sites, such as the root or plexus (e.g., Erb’s point), are often unreliable. When stimulating proximally, it is often difficult to ensure supramaximal stimulation or to limit stimulation to one nerve. Alternatively, evaluation of late responses can provide useful data regarding the proximal portions of nerves. The two most commonly evaluated late responses are the F wave and H-reflex (Fig. 232-7).
Repetitive Stimulation
Repetitive stimulation is used to diagnose abnormalities of the neuromuscular junction, such as myasthenia gravis, botulism, Lambert-Eaton myasthenic syndrome, and congenital myasthenia. The technique involves rapid, repetitive CMAP recordings from 2 to 50 Hz. Defects in neuromuscular transmission, especially at the postsynaptic site, lead to successive decrements in CMAP amplitude with stimulation at low frequencies of 2 to 3 Hz. An increase in CMAP amplitude at high frequencies such as 50 Hz is indicative of a presynaptic neuromuscular transmission defect, such as Lambert-Eaton syndrome. The principles underlying these abnormalities can be found in other textbooks.1,2
Needle Electromyography
Spontaneous Activity
Under normal conditions, when a patient is relaxed, muscle fibers are electrically silent, with no significant spontaneous muscle fiber action potentials. Different types of abnormal spontaneous muscle fiber action potentials can be seen and indicate specific abnormalities of the PNS (Table 232-1). The most common and significant spontaneous activities consist of fibrillation potentials and positive sharp waves (Fig. 232-8A and B). These are spontaneous action potentials from individual muscle fibers in response to either acute denervation or acute muscle fiber injury. Muscle disorders associated with these discharges include muscle fiber necrosis from muscle trauma, muscular dystrophies or inflammatory myopathies, and other muscle diseases, such as acid maltase deficiency or hyperkalemic periodic paralysis. Because fibrillation potentials and positive sharp waves are caused by the same group of PNS abnormalities, they usually occur together. Denervation of muscle results in fibrillation potentials and positive sharp waves within 2 to 3 weeks. These findings persist until the muscle fiber is reinnervated, usually within 3 to 4 months in mild injuries, or until the denervated muscle fiber undergoes complete atrophy after up to a few years of persistent denervation without reinnervation.
TABLE 232-1 Abnormal Spontaneous Muscle Fiber Action Potentials and Associated Peripheral Nervous System Abnormalities
| MUSCLE FIBER ACTION POTENTIAL | ABNORMALITY |
|---|---|
| Fibrillation potential | Acute denervation, acute muscle fiber necrosis |
| Positive sharp wave | Acute denervation, acute muscle fiber necrosis |
| Complex repetitive discharge | Chronic denervation, chronic muscle fiber necrosis |
| Fasciculation potential | Normal finding, motoneuron disease, radiculopathy, neuropathy |
| Myotonic discharge | Myotonic dystrophy, myotonia congenita, paramyotonia |
| Myokymic discharge | Radiation plexopathy or myelopathy, multiple sclerosis, brainstem glioma with facial myokymia |
| Cramp discharge | Normal finding, motoneuron disease, radiculopathy, neuropathy |
| Neuromyotonic discharge | Isaac’s disease, neuropathy |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree