Fig. 4.1.
(a) A schematic of the 5′ end of the Gabra1 locus. As described in the text, a yeast-mediated recombination step replaced the genomic region to be deleted with an amplified yeast selection cassette. The final targeting vector was completed by replacing the yeast selection cassette with a LacZ/neomycin selection cassette (14). (b) The 5′ external probe P1/P2 was used to screen and confirm Bgl I digested ESC genomic DNA. The expected wild type and targeted band sizes are shown in a targeted ESC clone and a WT clone. (c) PCR genotyping of tail DNA was used to identify littermates as either WT (+/+), with a single PCR product of 252 bp, a KO (−/−) with a single targeted band of 315 bp, or a Het (+/−) with both bands. (d) Complete loss of Gabra1 protein was confirmed by Western blot analysis of brain membrane protein extract using a commercial anti-GABAA alpha1 antibody. Protein Gabra1 was detected as an apparent 50 kDa band in the wild type (+/+) which was absent in the KO (−/−) mouse.
Together with two other lines of Gabra1 KO mice previously described (9, 10), a comparison of the targeted exons and the genetic background of the three KO lines are summarized in Table 4.1.
Table 4.1
Summary of generation of the three lines of knockout mice made at the Gabra1 locus
Institutions KO mice originally generated | Deleted exon and disrupted protein domain | Genetic background | References |
|---|---|---|---|
Georgetown University Medical School | Exon 9 encoding the second and third transmembrane domains | C5 7BL/6J, 129/Sv/SvJ, and FVB/N | (10) |
Merck Sharp and Dohme Research laboratory | Exon 5 encoding the middle part of the ligand binding domain | ∼75% C57BL6 and ∼25% 1 29SvEv | (9) |
Lexicon Genetics Incorporated | Exon 3 encoding the front part of the ligand binding domain | C57BL/6 (albino) and 129/SvEvBrd | Own data |
All the mice used in the experiments were cohorts from F2 to F4 generations bred on a mixed albino C57BL/6J-Tyr c-Brd x 129S5/SvEvBrd genetic background (15, 16). The Lexicon albino C57BL/6J-Tyr c-Brd strain is identical to the commercially available C57 non-albino strain (C57Bl/6J from Taconic Farms, Inc.). The two C57 strains differ only by the spontaneous mutation that arose in the c locus, thus resulting in albinism. The detailed description of the albino strain can be found in MGI database (Phenotypic Allele Detail, C57/BL6J-Tyrc-Brd
http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=alleleDetail&key=43586). An albino strain was originally chosen as a background strain for Lexicon Pharmaceuticals knockout mice because chimeras derived from albino embryos allow for a more accurate measurement of chimerism than non-albino black strain.
All experiments were carried out with protocols approved by The Institutional Animal Care and Use Committee of Lexicon Pharmaceuticals.
The complete deletion of Gabra1 protein in our Gabra1 KO mice was confirmed by western blot analysis using anti-GABAA alpha1 antibody (1:500, Sigma, G4416) on wild type and KO mouse brain membrane proteins. The Gabra1 was detected as a protein band of 50 kDa apparent molecular weight (Fig. 4.1d) in the wild type mouse and was absent in the Gabra1 KO mouse.
3 Mutation of α1 Subunit Results in Altered Behavior
3.1 General Behavior
We evaluated Gabra1 KO mice in a standardized phenotyping battery. This comprehensive phenotypic analysis also included a set of behavioral tests derived from the Irwin screen to determine the gross sensory and motor deficits (17). As part of this screen general physical health and empty cage spontaneous behaviors were observed by placing the mouse in an empty home cage for 60 s. Afterwards, the mouse was lifted by the tail and observed for limb splaying and forepaw and hindpaw clutching for 20 s.
Our KO mice were smaller in body weight and length, but viable and fertile. There were no differences between the KO and WT control mice in motor coordination, sensorimotor gating, depressive-like behavior, or learning and memory as assessed by inverted screen, pre-pulse inhibition, tail suspension, and trace fear conditioning assays, respectively (Table 4.2).
Table 4.2
Initial behavioral characterization of Gabra1 KO mice
WT | KO | |
|---|---|---|
Open Field | ||
Total distance (cm) | 1,835 ± 440 (28) | 1,404 ± 897 (29)* |
Center time | 352 ± 165 (28) | 198 ± 216 (29)** |
Rearing | 52 ± 31 (28) | 18 ± 16 (29)*** |
Basal body temperature | 36.0 ± 0.61 (29)*** | |
Stress-induced hyperthermia | 1.31 ± 0.5 (14) | 1.49 ± 0.5 (14) |
Inverted Screen | ||
Fell down (ratio) | 0/28 | 2/29 |
Climbed up (ratio) | 18/28 | 16/29 |
Marble burying | 10.6 ± 6.2 (28) | 1.3 ± 3.5 (29)*** |
Acoustic startle response | 420 ± 270 (28) | 783 ± 462 (29)*** |
PPI (%) | ||
pp4 | ||
pp8 | ||
pp12 | ||
pp20 | ||
Tail Suspension | ||
Immobility (sec) | 103 ± 52 (27) | 78 ± 74 (29) |
Hot Plate | ||
Latency (sec) | 10.4 ± 3.5 (28) | 15.1 ± 6.8 (29)** |
Trace Fear Conditioning | ||
Pre-CS freezing | 10.4 ± 8.4 (28) | 15.5 ± 19.1 (29) |
Post-CS freezing | 26.5 ± 19 (28) | 26.9 ± 22 (29) |
Difference freezing | 16.1 ± 16.7 (28) | 11.4 ± 16.2 (29) |
In the Irwin-derived initial behavioral screen, we observed tremor in Gabra1 KO mice as they moved freely about their cages, consistent with previous reports (9, 18). Despite the tremor, Gabra1 KO performed normally in an inverted screen assay that assesses motor strength and coordination (Table 4.2). In other reports, Gabra1 KO mice were reported as normal in swimming and balance beam tests (9). Variable results have been reported for performance on the rotarod (see Table 4.3 for comparison of phenotypes and corresponding references). Kralic et al. reported that Gabra1 KO mice fell off sooner in the accelerating rotarod test (18), while there was no effect of genotype when the speed was held constant within trials (with a stepwise increase in speed over trials) (9).
Table 4.3
Phenotypic comparison of different lines with mutations made at the Gabra1 locus
Phenotype | Vicini | Sur | H101R | Lexicon |
|---|---|---|---|---|
KO viability | Reduced (F2); normal (F5-7) (9) | Normal | ||
Size of KO | Reduced (F2) (56) | Reduced during postnatal development (F2); normal (F3-7) (9) | Reduced (males), F2-F4 | |
Intention tremor | Observed in KO (F2) (17) | Observed in KO (F2) (9) | Observed in KO (F2-F4) | |
Open field activity (OFA) | No effect of genotype (F5-7) (6) | No difference in vehicle-treated mice (11) | KO decreased activity and exploration (circadian dependent) | |
Motor performance | KO impaired performance (rotorod; F2) (17) | No genotype effect (balance beam(F2), swimming (F2), and rotorod(F4)) (9) | No noted difference in vehicle treated mice (horizontal wire test,rotarod) (12) | No genotype effect (inverted screen(F2)) |
Anxiety | No effects of genotype (elevated plus maze) (F5-7) (6) | No noted difference in vehicle treated mice (light/dark choice and elevated X maze) (12) | Decreased lighted measures (platform), decreased center measures(OFA), decreased marbles buried (MB) | |
Zolpidem | KO decreased sensitivity (LORR) (6) | KO decreased sensitivity (LORR) (58) | KO decreased sensitivity (OFA, pentylenetetrazole test) (11) | KO decreased sensitivity (open field and LORR) |
Diazepam | KO increased sensitivity (open field, LORR, and elevated plus maze) (6) | |||
Ethanol | KO reduced preference (2-bottle choice task), and increased sensitivity to the motor stimulating effect (open field) (7) | KO decreased sensitivity (LORR) (58) | No effect of genotype (LORR) (12) | KO decreased sensitivity (LORR) |
3.2 Anxiety-Related Behaviors
Several phenotypes were detected in assays with predictive validity for anxiolytic compounds, namely the marble burying (19–21), platform (22), and open field tests (23–26). One of the strongest phenotypes observed in the first cohort of mice was in the marble burying test. The marble burying (MB) test for anxiety-related and compulsive-like behaviors utilizes novelty-induced digging to assess the behavioral state of the subject and was performed as previously described in (27). Briefly, mice were individually placed into cages filled to a depth of 5 cm with clean bedding and 25 identical marbles evenly spaced across the bedding surface. After 30-min testing, each mouse was returned to its home cage and marbles covered by bedding 2/3 or more were scored as buried by an experimenter blind to genotype. There were no effects of sex or genotype by sex interaction; therefore, an unpaired t-test was used for all statistical analysis of MB. As seen in the Table 4.2, initial testing in MB revealed that Gabra1 KO mice buried less marbles when compared with WT cohort mates (t55 = 7.0; P < 0.001, Fig. 4.2a). Follow-up testing on a new cohort of Gabra1 mice produced results similar to the initial screening (Fig. 4.2b); Gabra1 KO again buried significantly less marbles than WT mice (t37 = 4.3; P < 0.001). The marble-burying assay is known to detect the activity of antidepressant and anxiolytic compounds, but does not always reveal a therapeutic direction (19–21, 28). Therefore, the significantly decreased digging behavior that we observed only provides an indication of altered anxiety behavior.
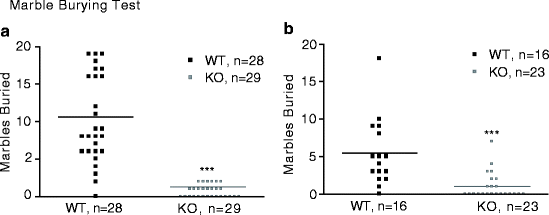

Fig. 4.2.
Marble burying in Gabra1 KO mice. Number of marbles buried is shown in graphs with group means displayed as solid lines. WT (n = 28, black symbols); KO (n = 29, grey symbols). Gabra1 KO mice buried less marbles compared to WT cohort mates. (a) Initial testing; (b) Follow-up replication testing. ***P < 0.001 – genotype effect.
We evaluated locomotor activity of Gabra1 KO and WT mice in the open field (AccuScan Instruments Inc., Columbus, OH, USA; 42 × 42 × 30 cm3) as described in (27, 29). Center time and center-to-total distance ratio have been used as indicators of unconditioned (trait) anxiety (23–26). Published studies of Gabra1 KO mice have reported inconsistent effects using these measures (Table 4.3). In our initial characterization of the Gabra1 KO, we found decreases in locomotor activity, center measures (center time and center to total distance ratio) and exploratory behaviors (rearing and hole poke) in the open field (Table 4.2).
Having observed altered anxiety-like phenotypes in both MB and OF, we followed up with a more specific anxiety assay, the platform test. The platform test (an analog of the light/dark test) was performed as described in details in (22). In brief, a platform was positioned in the back right corner of the Digiscan open-field apparatus (AccuScan Instruments Inc., Columbus, OH, USA; 42 × 42 × 30 cm3). The animals were placed under the platform. Duration and distance in specific areas (under or outside the platform) were calculated using Versamap software (Accuscan Instruments). The platform test was conducted on a new cohort prior to the mice having any testing experience. The main measures of anxiety behavior in the platform test are time in the lighted area, i.e., outside of platform shelter, and the ratio of lighted to total area distance traveled (ld/td). Male Gabra1 KO mice spent less time in the lighted area (Fig. 4.3a) compared to male WT mice (t 38 = 3.0; P < 0.01). Male Gabra1 KO mice also exhibited a decreased ld/td ratio relative to male WT mice (t 38 = 4.0; P < 0.001, Fig. 4.3b). These findings of decreased time and distance spent in the lighted region in male Gabra1 KO mice are indicative of an anxiogenic phenotype. There was a nonsignificant trend in female Gabra1 KO mice toward reduced time spent on the lighted side when compared with female WT mice (t 37 = 1.5; P = 0.13, Fig. 4.3c). There was a nearly significant trend for decreased ld/td ratio in female Gabra1 KO mice (t 37 = 1.96; P = 0.05, Fig. 4.3d). Overall, Gabra1 deletion induced an anxiogenic-like phenotype in both male and female mutant mice in the platform test.
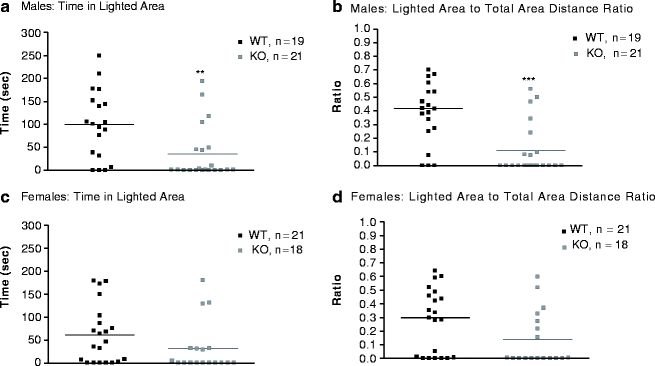

Fig. 4.3.
The Platform test in Gabra1 KO mice. Male WT (n = 19, black symbols); male KO (n = 21, grey symbols); female WT (n = 21, black symbols); female KO (n = 18, grey symbols). (a) Time in lighted area in males; (b) Light to total distance ratio in males; (c) Time in lighted area in females (P = 0.13); (d) Light to total distance ratio in females (P = 0.05). **P < 0.01, ***P < 0.001 – genotype effect.
The platform, open field, and marble burying tests are measures of unconditioned anxiety. We also tested mice in fear-potentiated startle (FPS), a test of conditioned fear and anxiety that is sensitive to amygdala damage and GABA mediated drugs (30). In the FPS assay, a fear reaction to a discrete conditioned stimulus is measured rather than a response to an unconditioned fear-provoking unfamiliar environment as in exploration-based paradigms like the plus-maze or light-dark tests. Previously, we have reported a dose-dependent decrease in FPS with the GABAA agonist chlordiazepoxide (31). An advantage of the FPS test is that it is not influenced by locomotor activity. The FPS test allowed us to examine if Gabra1 KO mice exhibited alterations in learned fear, often referred to as state anxiety, as opposed to unconditioned anxiety, commonly referred to as trait anxiety (32). Fear potentiated startle (FPS) was assessed using acoustic startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) for mice as described in (31). There were no significant differences due to genotype in FPS. Importantly, both WT and KO mice exhibited significantly higher startle in tone trials relative to no-tone trials, (F (1,144) = 8.6, P < 0.01, Fig. 4.4). This indicates that both WT and KO mice acquired conditioned fear to the tone stimulus and suggests that the conditioned fear is not affected by genetic disruption of Gabra1. Interestingly, Gabra1 KO mice exhibited significantly increased startle reactivity (F (1, 144) = 10.5, P < 0.01) in FPS. We also observed significantly increased startle reactivity in Gabra1 KO in PPI testing (Table 4.2). Additional evidence of Gabra1 involvement in startle behavior was reported by Homanics et al., who observed increased startle reactivity in Gabra1 homozygous knock-in mice (33). Startle reactivity has been used as a measure of unconditioned anxiety (34). Therefore, this result further substantiates the findings in marble-burying, platform, and open-field assays.
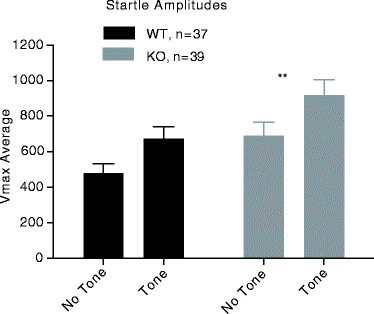

Fig. 4.4.
Fear potentiated startle response in Gabra1 KO mice. Mean response ± S.E.M. WT (n = 37, black bars); KO (n = 39, grey bars). Gabra1 KO mice had significantly increased overall startle compared to WT mice, **P < 0.01 – genotype effect.
Our results show that Gabra1 KO mice are not impaired in FPS, an assay with a learning component, suggesting that this subunit is likely not critical for learned anxiety or classical fear conditioning. We also have not observed differences between genotypes in trace fear conditioning. Therefore, while it is unclear the specific conditions Gabra1 may modulate anxiety-mediated learning and memory, our study strongly suggests that Gabra1 is particularly crucial for trait anxiety and not important for conditioned anxiety. It is interesting to note that Dixon et al. (2008) report that genetic disruption of Gabra2 impairs conditioned anxiety (35).
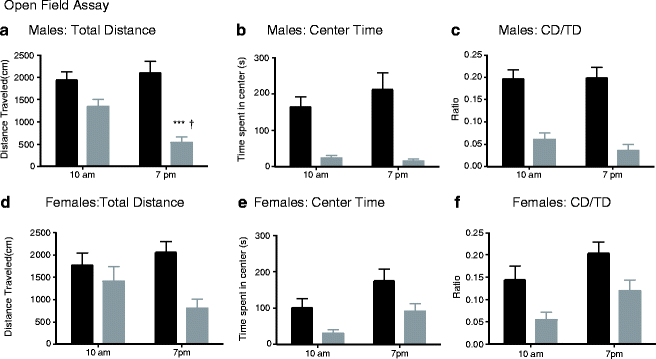
3.3 Circadian Influences on Behavior
Circadian influence on the GABA system has been well documented in mice and men (36, 37). We have followed this up using two additional cohorts. We tested mice in the open field at 10 am and 7 pm to determine the baseline differences between genotypes as well as circadian influences on activity and anxiety-related behaviors. When mice were tested during our normal testing hours (10 am), there was a significant effect of genotype on center time [F (1, 70) = 30.5, P < 0.001], and center to total distance ratio [F (1,70) = 29.9, P < 0.001] with decreases in these measures in KO mice (Fig. 4.5). The effect of genotype on total distance approached, but did not reach significance [F (1, 70) = 3.9, P = 0.051]. When tested at the beginning of the dark period (7 pm), there were significant effects of genotype on center time [F (1, 48) = 20.6, P < 0.001], center to total distance ratio [F (1, 48) = 31.4, P < 0.001], and total distance [F (1, 48) = 43.3, P < 0.001] with all measures decreased in the KO.