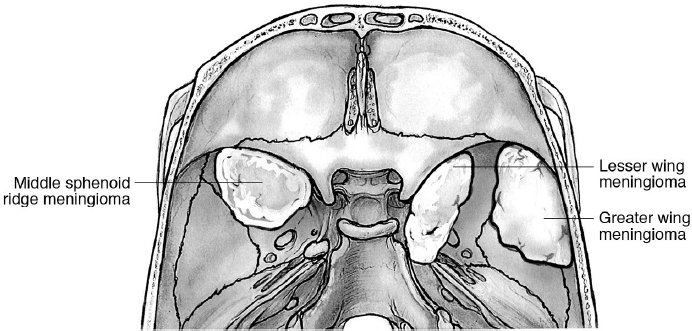
21 Middle Skull Base Surgery Meningiomas involving the sphenoid bone account for 11 to 18% of all intracranial meningiomas.1–3 The classification of the sphenoid meningiomas is based on their anatomic localization on the sphenoid wings (Fig. 21.1). The most common ones arise from the lesser wing; those on the greater wing are referred to as lateral sphenoid wing meningiomas and middle fossa meningiomas, although the size often blurs the distinction. Colloquially, the wings are incorrectly referred to as one wing, and the tumors are classified as those that occupy the medial, middle, or lateral component of the wing, or they may involve the orbit as spheno-orbital meningiomas. Other nomenclature: • Outer third meningiomas, middle third sphenoidal wing meningiomas, clinoidal, and spheno-orbital meningiomas. Outer third of the sphenoidal ridge meningiomas: “pterional” meningiomas are essentially convexity meningiomas. • Middle sphenoidal wing meningiomas: also called “alar” meningiomas. • Clinoidal meningiomas: in the region of the anterior clinoid process (ACP) or the “medial” sphenoid wing. For the anatomic complexity of the ACP, clinoidal meningiomas have been classified separately4 (see below). • Spheno-orbital meningiomas: Prominent bone invasion and hyperostosis characterize these tumors that essentially involve the orbital walls, giving rise to neuro-ophthalmologic or cosmetic problems (e.g., exophthalmos, proptosis). The subdural component of the tumor may be globular or may be quite thin and almost nonapparent in an “en-plaque” growth pattern. In any patient with exophthalmos and hyperostosis of the orbital walls, look for an en-plaque tumor; there will usually be one. The en-plaque growth pattern is defined as follows: • Five categories8,9: A, clinoidal tumors; B, en plaque meningiomas with hyperostosis of the sphenoid bone; C, large invasive tumors of the sphenoid ridge; D, middle sphenoid ridge; E, lateral sphenoid ridge. • According to the invasion of the surrounding structures10: A, lateral sphenoid wing, middle sphenoid wing, medial sphenoid wing with or without cavernous sinus (CS) infiltration; B, en-plaque meningiomas with or without CS infiltration; C, purely intraosseous tumors. Three types4: • Type I: medial to the ACP, extra-arachnoidal, arising from the subclinoidal dura at the level where the internal carotid artery (ICA) from its subdural component enters into the arachnoidal cisternal space. • Type II: arising superolaterally to the ACP; growing in the arachnoidal plane around the carotid cistern; separated then from the ICA and optic nerve by arachnoid membranes. This is the most common type. • Type III: arising from the optic foramen region and extending into it (giving rise to early ophthalmologic disturbances because of invasion of the pial layer). Be aware of the risk of visual loss and ICA injury when complete resection is attempted. Four types, based on the intraorbital tumor localization: • I, superolateral; II, inferomedial; III, orbital apex; IV, diffuse11 The signs and symptoms are related to the size of the meningioma and the anatomic relationships. • Exophthalmos: spheno-orbital tumors, hyperostosis of the orbital walls • Often only headache or vertigo for asymptomatic tumors • Blindness, optic nerve atrophy, visual field deficits (more typical for the clinoidal and spheno-orbital meningiomas) • Diplopia, oculomotor or trigeminal deficits: in cases of involvement of the CS and/or orbital fissures or intraorbital invasion • Cosmetic disturbances: pterional tumors invading the bone or temporal muscle • Major and minor neurologic deficits (e.g., aphasia, hemiparesis, personality changes, memory loss, seizures) due to large tumors compressing the surrounding brain (especially in large pterional meningiomas) • Seizures Computed tomography (CT) is essential for defining hyperostosis. Magnetic resonance imaging (MRI) with fat suppression is necessary for detecting intraorbital extension of the tumor. Computed tomography angiography (CTA), or angiogram, may be required to define the relationships of the tumors with the ICA and the middle cerebral artery (MCA). Balloon test occlusion may be required when involvement of the ICA is observed and a more aggressive surgical treatment is planned. Sphenoid meningiomas always require a strict pre- and postoperative neuro-ophthalmologic follow-up. The medial ones should have an endocrinologic assessment, especially in patients of childbearing age. Radiological follow-up (“scan and see”) is appropriate in asymptomatic patients with small tumors. In symptomatic patients, surgery is recommended especially for healthy and younger patients. Early and aggressive surgical treatment has been advocated in medial sphenoid wing meningiomas for maximizing the outcome and for vision preservation.12 • Radiotherapy (RT) and/or radiosurgery (RS) are valid alternatives, as well as subtotal/partial resection followed by RT/RS. RS is only used in those cases with at least 2 to 3 mm between the lesion and the optic nerve and chiasm so as to keep doses below 8 Gy. See also Chapter 30. Intraoperative neuromonitoring is not always required or useful, but electromyogram (EMG) of the oculomotor muscles may be of help for tumors with intraorbital or CS involvement (see page 90). 1. Pterional/frontotemporal craniotomy is performed based on the size of the tumor, with an additional orbitocranial (OC) or orbitozygomatic (OZ) osteotomy in order to further expose the anterior and middle cranial fossa, and to avoid brain retraction (see also Fig. 14.8, page 354). 2. Lateral supraorbital approach may be indicated. 3. Lateral transzygomatic approach may be indicated in some cases, such as for mobilization of the entire zygoma, leaving it pedicled on the masseter muscle.13 Lateral sphenoid bone and temporal bone can be hypertrophic and hypervascular. Performing a craniotomy on these bones is often dangerous and difficult. In such situation, drilling the bones is a valid alternative to the craniotomy.14 Fig. 21.3 Surgical approaches to the sphenoid tumors. FT, frontotemporal; OC, orbitocranial; OZ, orbitozygomatic. Surgical Anatomy Pearl Study meticulously the preoperative images for assessing the relationships. A true invasion of the MCA wall is very rare.4 Because the arachnoid is usually preserved by the tumor, stay outside the arachnoid and do not disturb the MCA or its branches. If the subarachnoid space must be opened, follow the branches of the MCA from the lateral aspect to the medial aspect. Try to preserve each of the small lenticulostriate arteries, which are very fragile and can be encased and stretched by the tumor. Pearl Portions of the tumor involving the CS are better treated by RS/RT, which have better results for ophthalmoparesis and carotid artery preservation.10,15–19 Surgical Anatomy Pearl In general, the tumors are extraconal and extra-periorbital. • Types I and III are the most difficult to remove due to their tight relationship with the internal carotid artery and the lack of a true arachnoidal plane. • Type II has a higher chance to be totally removed with less risk of injuring the ICA and the optic nerve. • The goal of surgery in type II and III clinoidal meningiomas is total resection, but subtotal resection may be required in type I (and III, in some cases) clinoidal meningiomas, in order to avoid excessive risk of injury of the ICA.20 Surgical Anatomy Pearl Do not coagulate around the optic nerve; every small oozing has to be controlled with Gelfoam or other hemostatic materials. Complete resection of these tumors may not be feasible, if the optic nerve dura propria is breached. The goal of surgery is tissue diagnosis, decompression to relieve proptosis and visual deficits, and resection of as much of the tumor as is safe to perform.21 Resected dura should be replaced by fascia lata, temporalis muscle fascia, or rarely pericranium. According to several neurosurgeons, these biological tissues remain the best material for duraplasty. Synthetic materials for duraplasty may be used as well. • Reinforce the closure with fibrin glue if there is a risk of cerebrospinal fluid (CSF) leak. • Periorbital defects should be repaired, with stitches or fascia lata/pericranium. • Orbital reconstruction is required only for massive destruction of the orbital walls (by the tumor and/or after surgery), causing enophthalmos. • Non-watertight duraplasty without orbital reconstruction is often satisfactory.14 Alar and pterional sphenoid meningiomas have a higher rate of total resection and better outcome in comparison with clinoid meningiomas (which have a higher risk of new or worsened neurologic deficits, occurring in 19%).25 Gross total resection is performed in 65 to 100% of alar sphenoid meningiomas3,25 versus 43 to 65% of clinoidal meningiomas.12,26 Visual improvement occurs in up to 64 to 80% of clinoidal meningiomas.12 • Worsening cranial neuropathy, especially in tumors invading the optic canal or CS (20% of cases)25 • CSF leakage: consider also the extremely rare iatrogenic CSF orbitorrhea (CSF leakage into the orbit) or oculorrhea (CSF leakage through the orbit to the exterior side)27 • Neurologic deficits: aphasia, hemiparesis (< 9%) • Seizures, especially with the alar meningiomas • Hydrocephalus (< 7%), stroke, wound infection, pneumonia These meningiomas arise from the fossa of the middle skull base, with no connection (or less than 25% connection) to the surrounding more typical localizations (sphenoid wing, CS, tentorium, lateral convexity).28–31 They account for 1.4% of all intracranial meningiomas.28 These meningiomas can be classified based on their connection/dural attachment.28 Signs and symptoms include headache, seizures, trigeminal deficits/neuralgia, cognitive decline, gait disturbances, hearing loss, and diplopia. The approaches include a frontotemporal craniotomy with or without OZ osteotomy, and a temporal craniotomy with or without a zygomatic osteotomy. Generally, these tumors are diagnosed when they become symptomatic due to their large size (> 3 cm), and the described overall surgical morbidity is 33% (new neurologic deficit in 20% of patients, worse neurologic deficit in 20%).28 Simpson grade 1 or 2 resection was achieved in 67% of patients in the only published series on purely middle fossa meningiomas.29,30 Review topographic anatomy, page 13. These tumors are listed in Table 21.1. The signs and symptoms include headache (generally nonspecific, sometimes localized to the vertex or fronto-orbital region) and visual abnormalities (blurred vision, decreased visual acuity, decreased night vision, tunneling of vision, campimetric deficits, papilledema).1 In giant tumors, the following signs are seen: compression of the frontal lobe, frontal lobe syndrome with mental deterioration, short-term memory loss, anosmia, and generalized seizures. Hypothalamus involvement is indicated by thermoregulatory imbalances. Cavernous sinus involvement is indicated by facial pain, oculomotor deficits and diplopia, and ptosis. Sphenoidal sinus erosion is indicated by CSF leak and epistaxis. Endocrinologic signs and symptoms are described in Chapter 9. Table 21.1 Lesions of the Sellar/Parasellar Region
 21.1 Meningiomas Involving the Sphenoid Bone
21.1 Meningiomas Involving the Sphenoid Bone
 General Information
General Information
Incidence
Definitions and Classifications
 En-plaque meningiomas: The definition is related to the growth pattern of the meningioma, which enables differentiation from the most typical “globular mass” meningioma. En-plaque meningiomas infiltrate the dura mater in a diffuse, sheet-like appearance, giving rise to marked hyperostosis. Microscopically, they are identical to other meningiomas. This growth pattern is seen in 2 to 9% of all meningiomas.5–7 The sphenoid wing is the typical localization of en-plaque meningiomas, although they have been described also in other skull regions. Their extensive origins can make them difficult to resect completely. The differential diagnosis includes other hyperostosis conditions: osteoma, Paget’s disease, fibrous dysplasia.
En-plaque meningiomas: The definition is related to the growth pattern of the meningioma, which enables differentiation from the most typical “globular mass” meningioma. En-plaque meningiomas infiltrate the dura mater in a diffuse, sheet-like appearance, giving rise to marked hyperostosis. Microscopically, they are identical to other meningiomas. This growth pattern is seen in 2 to 9% of all meningiomas.5–7 The sphenoid wing is the typical localization of en-plaque meningiomas, although they have been described also in other skull regions. Their extensive origins can make them difficult to resect completely. The differential diagnosis includes other hyperostosis conditions: osteoma, Paget’s disease, fibrous dysplasia.
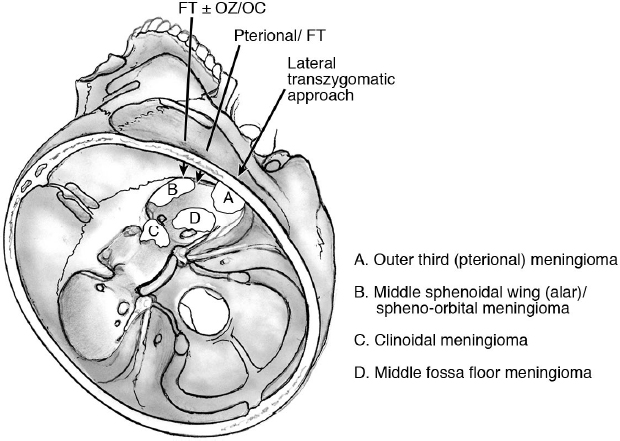
Classification Systems of Sphenoidal Meningiomas (Fig. 21.1)
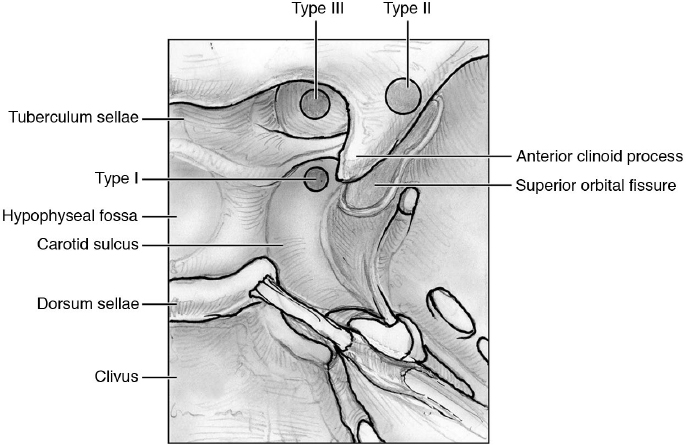
Classification of the Clinoidal Meningiomas (Fig. 21.2)
Classification of the Spheno-Orbital Meningiomas11
Signs and Symptoms
Diagnostic Workup
Treatment
Intraoperative Neuromonitoring
 Surgical Approaches (Fig. 21.3)
Surgical Approaches (Fig. 21.3)
 Perform an extradural dissection lateromedially to devascularize the tumor. Coagulation and division of the middle meningeal artery early are helpful in devascularizing the tumor.
Perform an extradural dissection lateromedially to devascularize the tumor. Coagulation and division of the middle meningeal artery early are helpful in devascularizing the tumor.
Lateral and Middle Sphenoidal Ridge Meningiomas

 Drilling invaded/hyperostosis bone may be performed after dura dissection to further devascularize the meningioma.
Drilling invaded/hyperostosis bone may be performed after dura dissection to further devascularize the meningioma.
 A curvilinear incision may be performed on the dura. Avoid early incision of the mediobasal dura in order to avoid epidural oozing in the subdural space.15
A curvilinear incision may be performed on the dura. Avoid early incision of the mediobasal dura in order to avoid epidural oozing in the subdural space.15
 An initial debulking of the tumor, also by means of the ultrasonic surgical aspirator, may be required for large tumors.
An initial debulking of the tumor, also by means of the ultrasonic surgical aspirator, may be required for large tumors.
 Identify and preserve all the branches coming from the MCA, which may appear “engulfed” by the tumor; the arachnoid layer is often preserved.
Identify and preserve all the branches coming from the MCA, which may appear “engulfed” by the tumor; the arachnoid layer is often preserved.
 After debulking, whenever required, perform a dissection of the tumor, keeping the arachnoid with the patient. This is mandatory in primary surgery, but it is more demanding (or not possible at all) in invasive meningiomas, recurrences, or after radiation therapy.
After debulking, whenever required, perform a dissection of the tumor, keeping the arachnoid with the patient. This is mandatory in primary surgery, but it is more demanding (or not possible at all) in invasive meningiomas, recurrences, or after radiation therapy.
 Any dura involved with the tumor (showing nodules, thickening, or dura looking invaded) should be resected. If available, do intraoperative histological analysis of dural margins to identify invasion.
Any dura involved with the tumor (showing nodules, thickening, or dura looking invaded) should be resected. If available, do intraoperative histological analysis of dural margins to identify invasion.

 For tumors involving the base of the middle cranial fossa, the dissection also has to be done around the foramina of the fossa. In cases of infratemporal invasion, the middle cranial fossa can be drilled as well.
For tumors involving the base of the middle cranial fossa, the dissection also has to be done around the foramina of the fossa. In cases of infratemporal invasion, the middle cranial fossa can be drilled as well.

 For the involvement of the CS, leave the tumor in situ and treat the residual with Gamma Knife or fractionated radiation if there is any concern about regrowth.
For the involvement of the CS, leave the tumor in situ and treat the residual with Gamma Knife or fractionated radiation if there is any concern about regrowth.

 In cases of invasion of the orbit, dissect and strip the periorbita from the tumor. Generally, the opening of the periorbita is not necessary, except in cases where the tumor is infiltrated by tumor nodules.15
In cases of invasion of the orbit, dissect and strip the periorbita from the tumor. Generally, the opening of the periorbita is not necessary, except in cases where the tumor is infiltrated by tumor nodules.15

 In cases of involvement of the superior orbital fissure, avoid excessive resection of the bony structures around it or transgression of the annulus of Zinn.
In cases of involvement of the superior orbital fissure, avoid excessive resection of the bony structures around it or transgression of the annulus of Zinn.
Clinoidal Meningiomas
 In such tumors, extradural anterior clinoidectomy may be indicated.
In such tumors, extradural anterior clinoidectomy may be indicated.
 The optic canal can be decompressed at this stage or after intradural debulking/resection of the tumor. If needed, resect the optic strut and the optic roof with drill (under copious irrigation) or with ultrasonic bone aspirator (see page 321).
The optic canal can be decompressed at this stage or after intradural debulking/resection of the tumor. If needed, resect the optic strut and the optic roof with drill (under copious irrigation) or with ultrasonic bone aspirator (see page 321).
 Open the dural layer over the optic nerve (compulsory in type III clinoidal meningiomas).
Open the dural layer over the optic nerve (compulsory in type III clinoidal meningiomas).
 After the extradural part, open the dura in curvilinear fashion, and perform arachnoid dissection to expose the margins of the tumor.
After the extradural part, open the dura in curvilinear fashion, and perform arachnoid dissection to expose the margins of the tumor.
Spheno-Orbital Meningiomas
 These cases are ideal for the two-piece OZ approach after a frontotemporal craniotomy. Alternatively, one can simply drill through the hyperostotic superior and/or lateral wall(s) of the orbit after a frontotemporal craniotomy and access the orbital tumor from this approach.
These cases are ideal for the two-piece OZ approach after a frontotemporal craniotomy. Alternatively, one can simply drill through the hyperostotic superior and/or lateral wall(s) of the orbit after a frontotemporal craniotomy and access the orbital tumor from this approach.
 Resect the lateral and superolateral orbital walls invaded by the tumor.
Resect the lateral and superolateral orbital walls invaded by the tumor.
 Perform a wide opening of the optic canal for tumors extending medially and affecting the optic nerve.
Perform a wide opening of the optic canal for tumors extending medially and affecting the optic nerve.

 For the rare tumor invading the inferior part of the orbit and/or nasoethmoidal cells, an anterior approach (either in a second surgical step),9 a combined infratemporal approach,22 or a transmalar or transzygomatic subciliary approach may be performed.23
For the rare tumor invading the inferior part of the orbit and/or nasoethmoidal cells, an anterior approach (either in a second surgical step),9 a combined infratemporal approach,22 or a transmalar or transzygomatic subciliary approach may be performed.23

 Combined approaches requiring transcranial approach and endonasal endoscopic approach may be required for multicompartmental sphenoid meningiomas.24
Combined approaches requiring transcranial approach and endonasal endoscopic approach may be required for multicompartmental sphenoid meningiomas.24

 For the rare tumors invading the maxillary/malar bones, craniofacial approaches have to be performed, and bone reconstruction is performed for cosmetic reasons.
For the rare tumors invading the maxillary/malar bones, craniofacial approaches have to be performed, and bone reconstruction is performed for cosmetic reasons.
 Reconstruction
Reconstruction
 Outcome
Outcome
 Complications
Complications
 Middle Fossa Floor Meningiomas
Middle Fossa Floor Meningiomas
Incidence
Classification
Signs and Symptoms
Surgical Approaches
Outcome
 21.2 Sellar/Parasellar Tumors
21.2 Sellar/Parasellar Tumors
 General Information
General Information
Sellar/Parasellar Tumors
Signs and Symptoms
Benign tumors | Pituitary adenomas, meningiomas (tuberculum sellae, diaphragma sellae, from other surrounding anatomic structures), craniopharyngiomas, schwannomas, lipomas |
Malignant tumors | Lymphomas, germ cell tumors, chordomas, chondrosarcomas, pituitary carcinomas, pituitary blastomas, metastases |
Infiltrative processes | Lymphocytic hypophysitis, sarcoidosis, tuberculosis, histiocytosis X |
Cysts | Rathke cleft cyst, arachnoid cyst, dermoid cyst |
Other lesions | Abscesses, arteriovenous fistulas, germinomas, hemangiomas, granular cell tumors, gangliocytomas, astrocytomas, hamartomas, aneurysms |
Diagnostic Workup
Computed tomography is essential for defining the sellar-parasellar and nasal anatomy, and above all for surgical planning. X-ray may show enlargement/erosion of the sellar region due to pituitary macroadenomas or meningiomas. MRI is essential for defining localization and involvement of the optic chiasm and frontal lobe edema (T2 sequences). MRI with fat suppression is useful for identifying tumor extension into the optic canal. CTA or, rarely, angiogram may be required to define the relationships of the tumors relative to anterior cerebral arteries, or to rule out the presence of aneurysms (see Chapter 5).
• Perform a neuro-ophthalmologic workup (see Chapter 10).
• Perform an endocrinologic workup for pituitary gland/hypothalamus functionality. Check for adiposity, subnormal temperature, slow pulse, and increased sugar tolerance, indicative of hypothalamic dysfunction.
• If an endoscopic transsphenoidal approach is planned, a full otolaryngological assessment with nasal endoscopy is needed to assess the sinonasal cavities for any concurrent pathologies (e.g., nasal polyps), anatomic variants from prior surgery (e.g., septal perforation), or any relative contraindications to endonasal surgery (e.g., narrow surgical corridor because of nasal vestibule stenosis or untreated sinusitis).
• Perform neuropsychological tests as well: Montreal Cognitive Assessment (MOCA), Mini–Mental State Examination (MMSE), or other tests for frontal lobe functionality and memory, which are useful above all for follow-up over time.
Treatment
Asymptomatic small tumors should be followed over time. Radiosurgery is an option for patients in whom surgery is not indicated or for tumor remnants/recurrences, especially in the case of pituitary adenomas that are clinically aggressive. Surgery remains the gold standard of treatment, particularly for symptomatic patients with signs of rapid visual deterioration.
 Pituitary Tumors
Pituitary Tumors
Epidemiology and Pathology
For epidemiology and pathology, see page 143; for endocrinology, see page 222.
Classification
Endocrinologic Classification
Pituitary tumors are classified as functional (secreting) or nonfunctional (nonsecreting).
Size-Related classification
Microadenomas are less than 10 mm in size, and macroadenomas are 10 mm or larger. Giant adenomas have a size > 40 mm or volume > 10 cm3.2
Histological and Immunohistochemistry Classification
Anatomic Classification
Pituitary tumors can be sellar, suprasellar and/or parasellar. They can invade the surrounding spaces, such as the sphenoid sinus and/or the CS (as shown radiologically or histologically).
• The Hardy classification system3,4 is described in Fig. 21.4.
The suprasellar extension of the tumor is not a criterion of invasiveness, because such extension may be related to the position of the adenoma within the normal pituitary gland, the size of the opening of the diaphragma sellae,5 or the presence or size of the arachnoidal subdiaphragmatic cistern.6
• A grade five has been added for distant spread via CSF or blood (pituitary carcinoma).7 The asymmetrical parasellar extension type E refers to the extracranial extradural invasion (into the lateral CS).7
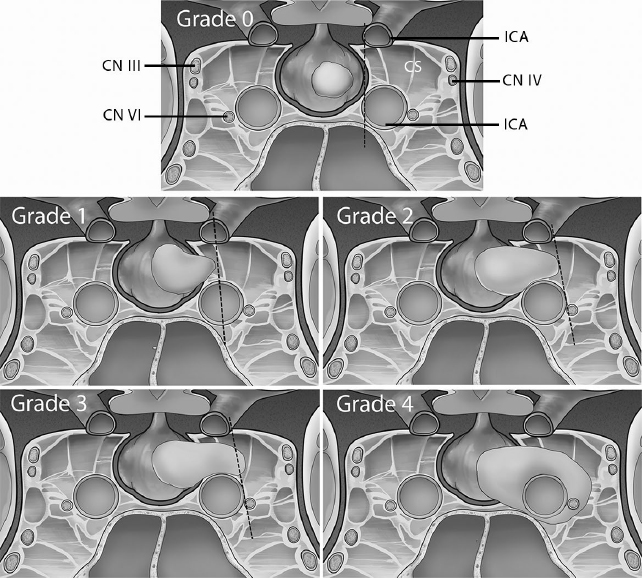
• The Knosp classification system8 (Fig. 21.5) is based on the invasion of the CS, with grades 3 and 4 considered to be true cavernous invasion.
Clinical Classification
Tumors are classified as typical adenomas and aggressive pituitary adenomas, the latter showing earlier and more frequent recurrences and possible resistance to conventional treatments, including radiotherapy.9
Surgical Indications for Pituitary Tumors
Indications include Cushing’s disease, acromegaly, prolactinoma not responsive to medical therapy, nonfunctioning tumors with mass effect on the optic system or beyond, and pituitary apoplexy with optic nerve or chiasm functional impairment.
• Pituitary tumors may require a multimodal treatment, especially the aggressive ones.
• See indications and outcomes of radiotherapy on pituitary tumors on page 774.
Approaches to Pituitary Tumors
Transcranial Surgery
Pituitary tumors, particularly the giant ones with large suprasellar components/invasion of the surrounding structures, may be treated by the standard transcranial approaches: pterional or subfrontal craniotomies (± OZ osteotomy), or rarely a subtemporal approach. Many of these patients can be treated by means of transsphenoidal approaches, particularly endoscopic ones.2
Transcranial surgery may be helpful if transsphenoidal surgery is considered difficult or contraindicated. For example, many surgeons in the past considered the conchal-type sphenoid sinus (see Fig. 2.14, page 53) as a relative indication for a craniotomy. However, with neuronavigation, this indication is becoming rare. Infections of the nose/paranasal sinuses, asymmetric extension of giant tumors around the parasellar compartments, some hourglass tumors (constrained into small openings of the diaphragm), and tumors with a very firm or fibrous consistency may still benefit from a craniotomy approach.
Endonasal Submucosal with Sublabial or Transseptal Approaches
See page 345.
Endoscopic Transsphenoidal Approach
In several centers, this approach is considered the gold standard, although not universally accepted as such.
Combined Approaches
Very stiff sellar tumors with large suprasellar components may require a combined approach, such as transsphenoidal approach for intrasellar component resection followed by the transcranial route. Also, giant multicompartmental tumors may require combined approaches (such as the pterional approach for lateral extension of the tumor, and the interhemispheric transventricular approach for resection of the intraventricular components).
 Endoscopic approach, technical nuances (see also Chapter 16):
Endoscopic approach, technical nuances (see also Chapter 16):
 A nasoseptal vascularized mucosal pedicled flap can be used in patients at risk for postoperative CSF leakage. It can also be used for bone coverage and to avoid excessive postoperative crusting.
A nasoseptal vascularized mucosal pedicled flap can be used in patients at risk for postoperative CSF leakage. It can also be used for bone coverage and to avoid excessive postoperative crusting.
 The approach may be performed via a single nostril or by using the four-hand bi-portal technique,10 although the biportal technique with a posterior septostomy and sphenoidotomy is used more commonly.
The approach may be performed via a single nostril or by using the four-hand bi-portal technique,10 although the biportal technique with a posterior septostomy and sphenoidotomy is used more commonly.
 The sphenoid sinus anatomic landmarks must be recognized (see Fig. 2.15, page 54). Removal of the sphenoidal septa enables wider surgical access. It is important to note the location of the septa on preoperative imaging and to connect these locations to intraoperative findings in order to clearly correlate and validate the intrasphenoidal surgical anatomy, including the position of the ICA. Stripping of the mucosa may also help in recognizing the landmarks.
The sphenoid sinus anatomic landmarks must be recognized (see Fig. 2.15, page 54). Removal of the sphenoidal septa enables wider surgical access. It is important to note the location of the septa on preoperative imaging and to connect these locations to intraoperative findings in order to clearly correlate and validate the intrasphenoidal surgical anatomy, including the position of the ICA. Stripping of the mucosa may also help in recognizing the landmarks.
 The bone of the sellar floor may be (1) thinned by using a diamond drill and then fractured; (2) removed with punches; or (3) removed en bloc by using chisels and a mallet if the sellar floor is thin in the periphery of the bony margins. Save the bony flap for reuse in the reconstruction of the sellar floor after the tumor resection. The margins of the sellar opening may be widened using punches, in order to identify the “four blues”, that is, the two CSs bilaterally and the superior and inferior intercavernous sinuses (the latter, whenever present) on the superior and inferior margins. If available, use a micro-Doppler probe for detecting the ICA. Doppler may also identify the superior and inferior intercavernous sinuses.
The bone of the sellar floor may be (1) thinned by using a diamond drill and then fractured; (2) removed with punches; or (3) removed en bloc by using chisels and a mallet if the sellar floor is thin in the periphery of the bony margins. Save the bony flap for reuse in the reconstruction of the sellar floor after the tumor resection. The margins of the sellar opening may be widened using punches, in order to identify the “four blues”, that is, the two CSs bilaterally and the superior and inferior intercavernous sinuses (the latter, whenever present) on the superior and inferior margins. If available, use a micro-Doppler probe for detecting the ICA. Doppler may also identify the superior and inferior intercavernous sinuses.

 Whenever present and required, coagulate the inferior intercavernous sinus.
Whenever present and required, coagulate the inferior intercavernous sinus.
 Open the dura-periosteum of the sellar floor to expose the tumor. The dura can be opened by making a quadrangular incision, a T incision, or a stellate incision (star shaped), in order to have access to the tumor.
Open the dura-periosteum of the sellar floor to expose the tumor. The dura can be opened by making a quadrangular incision, a T incision, or a stellate incision (star shaped), in order to have access to the tumor.
 Debulking of the tumor may be performed, especially in macroadenomas; otherwise an extracapsular dissection is suggested for an en-bloc resection, sparing the normal gland parenchyma. Start the dissection on the floor of the sella, proceeding laterally toward the CSs. The superior dissection has to reach the normal arachnoid of the diaphragma sellae. Use microcurettes of different sizes and angles to approach all the sides and components of the tumor (including the intracavernous components, whenever possible).
Debulking of the tumor may be performed, especially in macroadenomas; otherwise an extracapsular dissection is suggested for an en-bloc resection, sparing the normal gland parenchyma. Start the dissection on the floor of the sella, proceeding laterally toward the CSs. The superior dissection has to reach the normal arachnoid of the diaphragma sellae. Use microcurettes of different sizes and angles to approach all the sides and components of the tumor (including the intracavernous components, whenever possible).
 Use angled optics (≥ 30 degrees) for suprasellar components of the tumor.
Use angled optics (≥ 30 degrees) for suprasellar components of the tumor.
• Suprasellar components of the tumor might fall downward with the Valsalva maneuver.
• Control intratumoral bleeding with a hemostatic agent such as methyl-cellulose; local coagulation is generally not required. In large tumors, leaving a superior remnant may be risky because the remnant can hemorrhage postoperatively and cause hypothalamic compression. Usually, the bleeding from the tumor stops when the entire tumor has been removed. If it does not, look for more tumor or for injury to critical structures such as the venous sinus, the ICA, or a branch of the ICA, and consider packing it or ordering an angiogram when the bleeding is controlled.
Surgical Anatomy Pearl
The best way to avoid CSF leak is to keep the arachnoid intact. This can be achieved in over 95% of first-time surgeries.
Outcome
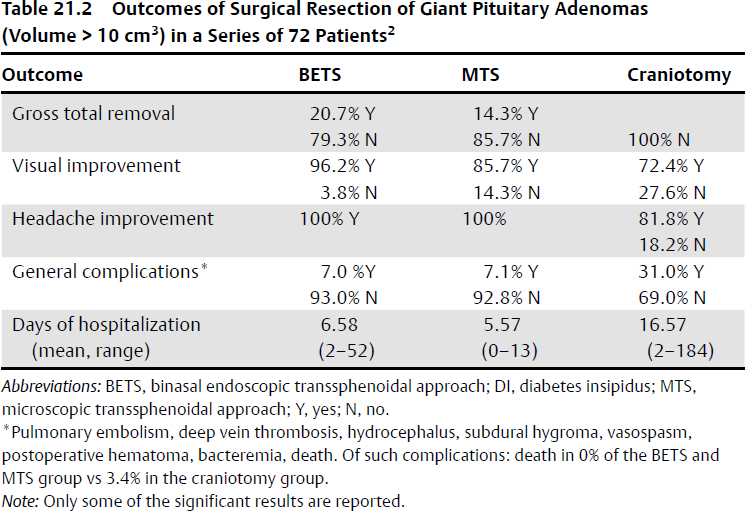
In microadenoma and non-giant macroadenoma, it is often possible to perform a total resection of the tumor. In giant pituitary adenoma, gross total resection has been achieved in 24% of patients, near-total resection in 17%, subtotal resection in 36%, and partial resection in 23%.11 A case series has shown a higher reduction of the volume of giant pituitary adenoma treated by means of the endoscopic transsphenoidal surgery than by means of a microscopic transsphenoidal or transcranial approach2 (Table 21.2).
• Visual deficit improvement in giant pituitary adenomas resection occurs at rates of 60 to 90%.2,11
• In pituitary adenomas with CS invasion, endoscopic endonasal resection is a feasible technique12,13 that enables resection of 85% of tumors with Knosp grade 1 or 2 and 67% of tumors with Knosp grade 3 or 4.14 The endonasal medial-to-lateral approach enables good resection of tumors in the medial aspect of the CS, but is not satisfactory in the lateral compartment. In fact, total/subtotal resection of the tumor in the most lateral part of the CS is generally not feasible by means of the endoscopic approach. In patients with acromegaly, CS invasion is the most significant predictor of unfavorable outcome, and aggressive removal of the invading tumor and of the medial wall of the CS, whenever feasible, is advocated by some authors to increase the remission rate.15 Remnants/recurrences in the CS after debulking endoscopic surgery should be evaluated for their hormonal production, growth, or potential for neurologic deficit. Despite the presence of remnants, many patients can still show endocrine remission.16 For those without a medical or surgical option, radiosurgery remains an effective alternative for tumor control and is also reasonable for endocrinologic control.
• The outcomes of transsphenoidal and microsurgical approaches for pituitary tumors are likely equivalent for experienced surgeons,17 although in some studies the endoscopic approach seems to provide an improved likelihood of tumor removal, and it is safer,18 especially for tumors within the CS.2,19,20 Some authors believe that vascular complications may be more prevalent in the endoscopic transsphenoidal technique,21 whereas others find no difference.5 The microscopic and endoscopic techniques provide similar outcomes in the treatment of nonfunctioning pituitary adenomas (at least in Knosp grade 0 to 2 tumor types).22
Complications of Pituitary Tumor Surgery
• The overall complication rate is 9%23; the surgical mortality is < 1%.
• CSF leakage occurs in 5% or less.23 A series has shown a higher intraoperative rate with the endoscopic technique than with the microscopic technique, with no difference in the incidence of postoperative CSF rhinorrhea.22 The risk of meningitis is < 2%.
• Visual deterioration occurs in 2%.
• Hypothalamic injury, hypopituitarism, diabetes insipidus (transient in < 5%, permanent in < 1%),24 CS nerves injury, brain/brainstem injury.
• Pneumocephalus (especially in the endoscopic approaches).
• Vascular complication (< 2% ICA injury; it is possible to lower it to 0% by using the intraoperative Doppler probe and micro-hook blade25). Rare cases of postoperative ICA vasospasm have been described in the event that subarachnoid bleeding is encountered.26
Surgical Pearl
Rescue in case of ICA injury: pack the defect with hemostatic material (Gelfoam, Surgicel), and transfer the patient to the endovascular suite for cerebral angiogram and endovascular repair. Proximal control of the cervical ICA in the neck is rarely required but can be lifesaving in the even that it becomes necessary to control bleeding.
• The endonasal approaches are related also to rhinological complications (epistaxis, hyposmia/anosmia, nasal crusting/synechiae, mucocele/pyocele, septal deviation).
 Craniopharyngioma
Craniopharyngioma
This is a benign epithelial tumor, World Health Organization (WHO) grade I, that is often totally or partially cystic, and typically is located in the sellar-suprasellar region. It arises along the vestiges of the stomodeal diverticulum, mostly at the level of the infundibulum, where squamous epithelial rests occur,27–29 or along the remnants of the primitive craniopharyngeal duct (nasopharynx, sphenoid bone, or predominantly intraventricular).27,30–33
Epidemiology and Pathology
Craniopharyngioma accounts for 1.2 to 5% of all intracranial tumors. The incidence is 0.5 to 2.5 cases per one million people per year.34 Craniopharyngioma is the most common tumor in children (5–10%).35 The incidence peaks between the ages of 5 and 15 years and between the ages of 45 and 60 years.36 The papillary subtype is more common in adults than in children. (See also Chapter 6, page 148).
Vascularization
Craniopharyngioma is vascularized by perforators from the ICA, posterior cerebral artery (PCA), anterior cerebral artery (ACA), and anterior communicating artery (AComA).
Clinical Presentation
• Endocrinologic dysfunction:
 Children: delay in puberty onset, short stature
Children: delay in puberty onset, short stature
 Children/adults: diabetes insipidus, hypothyroidism
Children/adults: diabetes insipidus, hypothyroidism
 In cases of intrasellar invasion with pituitary involvement: hypopituitarism, amenorrhea, galactorrhea, infertility
In cases of intrasellar invasion with pituitary involvement: hypopituitarism, amenorrhea, galactorrhea, infertility
• Neurologic deficits:
 Visual deficits: decreased vision, bitemporal hemianopia, optic nerve atrophy, papilledema (especially in hydrocephalus)
Visual deficits: decreased vision, bitemporal hemianopia, optic nerve atrophy, papilledema (especially in hydrocephalus)
 Signs and symptoms related to obstructive hydrocephalus/raised intracranial pressure: headache, nausea, vomiting
Signs and symptoms related to obstructive hydrocephalus/raised intracranial pressure: headache, nausea, vomiting
 Hydrocephalus is present in up to 48% of children38 and 13% of adults,39 and is generally caused by tumors with retrochiasmatic localization.
Hydrocephalus is present in up to 48% of children38 and 13% of adults,39 and is generally caused by tumors with retrochiasmatic localization.
 Craniopharyngioma is often diagnosed based on the related obstructive hydrocephalus, which is a surgical priority, above all in children. Behavioral disturbances: frontal lobe syndrome, memory loss, apathy, incontinence, hypersomnia, and Korsakoff syndrome (involvement of mammillary body and limbic system; rare)
Craniopharyngioma is often diagnosed based on the related obstructive hydrocephalus, which is a surgical priority, above all in children. Behavioral disturbances: frontal lobe syndrome, memory loss, apathy, incontinence, hypersomnia, and Korsakoff syndrome (involvement of mammillary body and limbic system; rare)
• Seizures are rare, but have been described after surgery.
Preoperative Assessment
Magnetic resonance imaging with and without gadolinium is essential to see the nature of the tumor, its cystic components, and the relations with the diencephalic structures. Magnetic resonance angiography (MRA) or CTA and rarely digital subtraction angiography (DSA) are used for demonstrating displacement/encasement of arteries. See also Table 5.4 on page 119.
• CT scan is performed to identify intralesional calcifications (present in 50% of adults and 100% of children)40,41 as well as the bony anatomy of the skull base in preoperative planning.
• Preoperative endocrinologic evaluation and postoperative follow-up assess pituitary gland and hypothalamus function/dysfunction and determine the need for replacement therapy. Diabetes insipidus is a presenting symptom in up to 38% of patients, rising to 70% after surgery. It has to be corrected in either case with vasopressin (see Chapter 9).
• Neuro-ophthalmological evaluation and postoperative follow-up are indicated.
• Neurobehavioral assessment and neuropsychological testing are indicated, particularly in children.
Differential Diagnosis
The differential includes sellar and suprasellar tumors, such as pituitary adenomas, Rathke cleft cysts, meningiomas, dermoids/epidermoids, arachnoid cysts, more rarely germinoma, hamartoma, abscess, inflammatory diseases, suprasellar aneurysms, and optic nerve or hypothalamic gliomas.
Anatomy-Based Classifications
• Based on the relationship with the diaphragma sellae, craniopharyngioma can be sellar (infradiaphragmatic), suprasellar (supradiaphragmatic), or both.
• Relative to the optic chiasm, craniopharyngioma can be prechiasmatic, subchiasmatic, or retrochiasmatic.
• Relative to the third ventricle, craniopharyngioma can be intra- or extraventricular, or below the ventricle.42
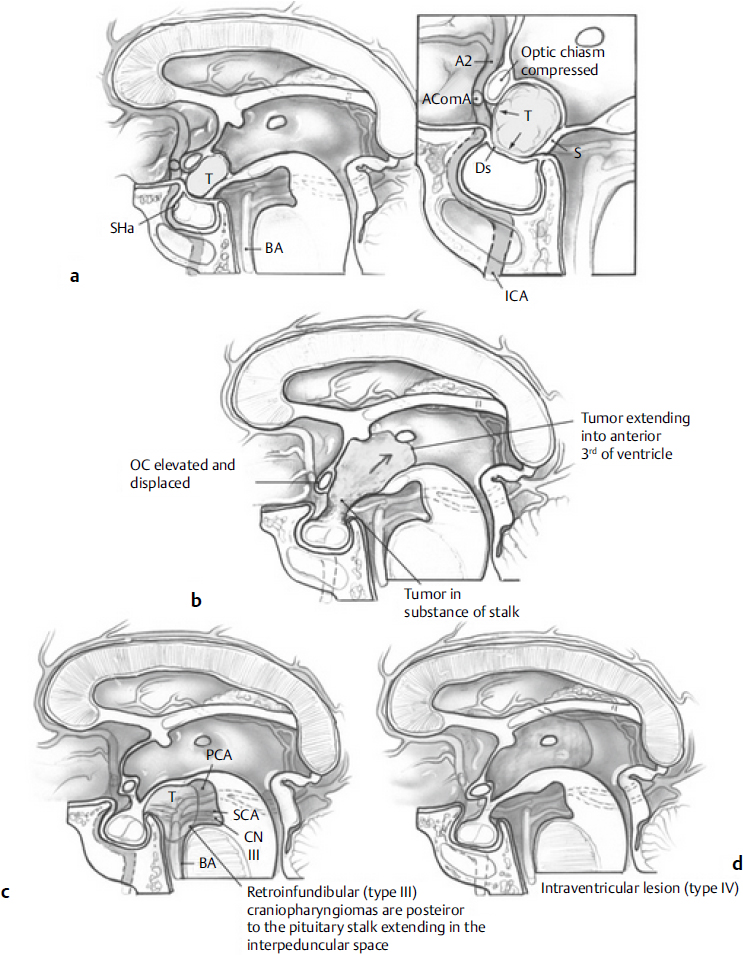
• There are six types43: (1) purely intrasellar-infradiaphragmatic; (2) intraand suprasellar, infra- and supradiaphragmatic; (3) supradiaphragmatic, parachiasmatic, extraventricular; (4) intra- and extraventricular; (5) paraventricular in relation to the third ventricle; and (6) purely intraventricular.
• Craniopharyngioma can be classified based on the suprasellar extension and infundibulum as follows44 (Fig. 21.6):
 Type I: preinfundibular
Type I: preinfundibular
 Type II: transinfundibular (with extension into the stalk)
Type II: transinfundibular (with extension into the stalk)
 Type III: retroinfundibular (extending behind the pituitary gland and stalk)
Type III: retroinfundibular (extending behind the pituitary gland and stalk)
 IIIa: extending into the third ventricle
IIIa: extending into the third ventricle
 IIIb: extending into the interpeduncular cistern
IIIb: extending into the interpeduncular cistern
 Type IV: isolated to the third ventricle and/or optic recess
Type IV: isolated to the third ventricle and/or optic recess
Surgical Approaches
Surgery is the primary treatment for the management of craniopharyngiomas. Debates exist regarding the advantages and disadvantages of minimal or maximal surgery. Proponents of the minimalist approach argue that total resection is associated with too high a risk, and it is best to treat only the symptomatic component as many times as is necessary to deal with the patient’s symptoms. Maximalists argue that the best approach in the long term is the safe removal of as much of the tumor as possible during the first operation, to decompress and to minimize recurrences. The maximalists also argue that this is safer than many repeated surgeries. Gross total, near total, or subtotal resection may be complicated by recurrences, particularly the development of cysts, which may be minimized with fractionated radiotherapy.45 Radiotherapy after resection is advocated because, after gross total resection, a mean of 21% recurrence has been described (65% in subtotal resection, 42% after partial resection46), which is decreased to 17% after gross total resection followed by radiotherapy (see also Chapter 30, page 791).45,47
Subfrontal Approach
This approach is indicated especially for suprasellar tumors in the presence of a postfixed chiasm. A translamina terminalis extension of the approach may be required to deal with the retrochiasmatic or intraventricular components48,49 and to facilitate access to the vertical components of the tumors. An OZ osteotomy may also be very helpful in reaching superiorly placed components of the tumor.
Frontolateral Approaches
The pterional or frontotemporal approach is one of the most used, often with its lateral supraorbital or supraorbital variants, giving access to the triangles of the sellar-parasellar region for removal of tumors between the optic nerves and the opticocarotid triangle or between the ICA and cranial nerve (CN) III. Although these triangles may be quite narrow, the tumor may widen the space between the ICA and optic nerve, thus providing access in certain cases. The frontolateral approaches enable doing a clinoidectomy and optic nerve unroofing, but if the tumor extends widely in a contralateral direction, the visualization of the optic nerve can be difficult. For small asymmetric tumors, the supraorbital keyhole approach has been suggested as well, but the approach is limited by the size of the frontal sinus, and there is limited vertical exposure.
Anterior Interhemispheric Approach
This approach is indicated for prechiasmatic tumors or those with large extensions into the third ventricle but that are primarily midline in nature.
Surgical Pearl
The frontolateral approaches may be extended with an OZ osteotomy, in order to give more basal access and less frontal lobe retraction to reach the superior aspects of the tumor. Some surgeons advocate the eyebrow approach using a mini-craniotomy and endoscope assistance for resection of the tumor.50–53
Subtemporal Approach
This approach is indicated for retrochiasmatic tumors with inferior or lateral extension to the middle fossa.54
Transpetrosal Approach
This approach is suggested for retrochiasmatic craniopharyngiomas, because it provides an upward projection to dissect the upper pole of the tumor and it facilitates visualization of the hypothalamus and pituitary stalk.55–57 Prolonged temporal lobe retraction is one of its limitations.
Extended Endoscopic Endonasal Approach
This approach is indicated for sellar/suprasellar tumors, with a larger component in the sella.58–61 It has the advantage of approaching the chiasm and AComA complex from below rather than from above. It requires meticulous dissection and patience but can be very effective.
Transcallosal and Transcortical Approaches
These approaches are used for pure intraventricular craniopharyngiomas or extraventricular tumors with large intraventricular components.
Intracavitary Therapy, Cyst Drainage
Purely cystic craniopharyngiomas may undergo sole intracystic aspiration via a catheter connected to an Ommaya reservoir, which is used for aspiration of the cystic liquid over time. A single aspiration is not suggested, considering the high likelihood of the cyst refilling. Moreover, the intracystic catheter can be used for the instillation of radioisotopes (32 phosphorus, 90 yttrium, 123 iodine, 198 aurum, 186 rhenium) to deliver a high local dose (~ 150 Gy) of radiation to the epithelial layer of the cyst, for sclerosing drugs (bleomycin),62 or for interferon-α.63 The catheter may be positioned by means of stereotactic- or endoscope-assisted guidance.
Surgical Pearls of the Transcranial Approaches
• Cerebrospinal fluid drainage may be required at the beginning of the operation, via ventricular or lumbar drain, above all in subfrontal approaches.
• An early debulking of the tumor and/or puncture of the cystic component may help in the dissection of the arachnoidal plane around the capsule of the tumor, especially at the level of the optic nerves, taking care to preserve the suprasellar vascularization (perforators to the optic chiasm and hypothalamus).
• In cases of anatomic variants of preor postfixed optic chiasm, or for displacement of the chiasm by the tumor, the planum sphenoidale can be drilled to create a larger space. In cases of violation into the sphenoid sinus from the planum, the skull base defect is sealed by suturing the pericranial flap (prepare it beforehand in the frontobasal approach) and by using fibrin glue and fascia lata.
• Every attempt to preserve the pituitary stalk should be made. The stalk can be difficult to visualize because of stretch/compression by the tumor, but the characteristic longitudinal striae of purplish portal blood vessels and the brownish tinge of the stalk are characteristic. In some cases the stalk has to be sacrificed for complete removal of the tumor. In such a case, transect the stalk as distally as possible in order to reestablish antidiuretic hormone (ADH) production.
Surgical Pearl
Avoid bipolar coagulation in the suprasellar cistern, whenever possible, in order to preclude vascular damage to the hypothalamus or the optic apparatus.
Other Surgical Approaches
Transbasal Subfrontal Translamina Terminalis Approach
This approach provides midline visualization of the optic chiasm and third ventricle, avoiding the blind spots of the supra- and retrosellar regions, as can occur in the frontolateral approaches.
• The subfrontal approach can be extended to transbasal or transglabellar via an orbitocranial osteotomy, with resection of the anterior wall of the frontal sinus, in order to provide more basal exposure with less retraction of the frontal lobes.48
 The tumor component in the third ventricle can be identified by opening the lamina terminalis. After opening, debulk the central part of the tumor (also by means of ultrasonic aspirator or by aspiration of the cystic components) and gently dissect the capsule from the ventricular walls, removing it through the lamina terminalis. Next, dissect and remove the components of the tumor in the infrachiasmatic and interpeduncular cisterns.
The tumor component in the third ventricle can be identified by opening the lamina terminalis. After opening, debulk the central part of the tumor (also by means of ultrasonic aspirator or by aspiration of the cystic components) and gently dissect the capsule from the ventricular walls, removing it through the lamina terminalis. Next, dissect and remove the components of the tumor in the infrachiasmatic and interpeduncular cisterns.
Extended Endoscopic Approaches
The endonasal route can be used for intrasellar subdiaphragmatic tumors64,65 as well as with supradiaphragmatic components (by means of extended approaches), for the management of remnants or recurrences.47 The extended endoscopic technique can be used also for intraventricular components, providing more direct access to subchiasmatic, retrosellar, and intraventricular components than with the transcranial approaches.47,66
• The transsphenoidal endoscopic approach is especially suggested for tumors with prechiasmatic or preinfundibular growth.44,67
• The position of the tumor in relation to the infundibulum may suggest extension of the approach.44 Type I lesions require a transplanum/transtubercular approach. Type II lesions require the superior intercavernous sinus split68 for an improved angle of attack. In type III tumors the infundibulum limits direct approach, which is why the pituitary gland and the stalk, according to some authors, have to be mobilized (pituitary transposition), in order to avoid neurovascular manipulation.44 Posterior clinoidectomy/superior clivectomy may be performed as well.
• A wide exposure in the extended approach is required for preserving the superior hypophyseal artery complex, which can be displaced from the tumor.
• Reconstruction techniques are mandatory for reducing the risk of postoperative CSF leak (see Chapter 16).
Disadvantages
Tumors isolated to the third ventricle or optic recess (type IV)44 may not be amenable to endoscopic resection. Moreover, recurrences/remnants of tumor previously treated by endonasal route may be more difficult to manage due to the presence of scars (i.e., vessels adherent to fascia lata, if previously used for the repair). As in all the transsphenoidal techniques, a relative contraindication is the presence of acute/subacute sinusitis.
Nuances of the Endoscopic Technique
• Lumbar drainage and the pedicled nasoseptal flap are highly recommended to reduce the risk of CSF leakage, above all the potential high-flow CSF leakages (i.e., opening of the ventricles).
• The superior intercavernous sinus is coagulated and divided to open the dura and access the suprasellar space.
Outcome
In a retrospective series of 100 craniopharyngiomas (79% purely infradiaphragmatic and 66% involving the supradiaphragmatic space), the endoscopic endonasal approach provided an overall gross-total removal of the craniopharyngioma in 70% of cases.69 Improvement to visual disturbances was observed in 75% of cases.
Surgical Complications
• Injury to the pituitary stalk
• Diabetes insipidus, which may occur in 47 to 93%39,70–72
• Panhypopituitarism: generally present before surgery; occurs in 50 to 100%, depending on tumor localization (there is a higher risk of panhypopituitarism in sellar tumors) and the preoperative pituitary function.72–74 In the case of normal preoperative pituitary function, new pituitary deficits occur in 58% of cases.72
• Hypothalamic injury: 40%75
• Vascular injury, blindness (which can be caused by optic nerve/chiasm direct injury or occlusion of the chiasmatic perforators): < 2%
• New cranial nerve palsy
• Pneumocephalus (especially in the endoscopic approaches)
• Infective or aseptic meningitis, the latter due to leakage of cystic material in the cisterns
Surgical Pearl
Leakage of cystic material in the cisterns may be avoided by aspiration of the intracystic fluid and continuous irrigation of the surgical cavity, for dilution of the tumor fluid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 Resect all the intraorbital–extra-periorbital components of the tumor. Nodules invading the periorbita may require opening of the periorbita. Invasion of the annulus of Zinn should not be approached aggressively in order to avoid the risk of cranial nerves injury.
Resect all the intraorbital–extra-periorbital components of the tumor. Nodules invading the periorbita may require opening of the periorbita. Invasion of the annulus of Zinn should not be approached aggressively in order to avoid the risk of cranial nerves injury.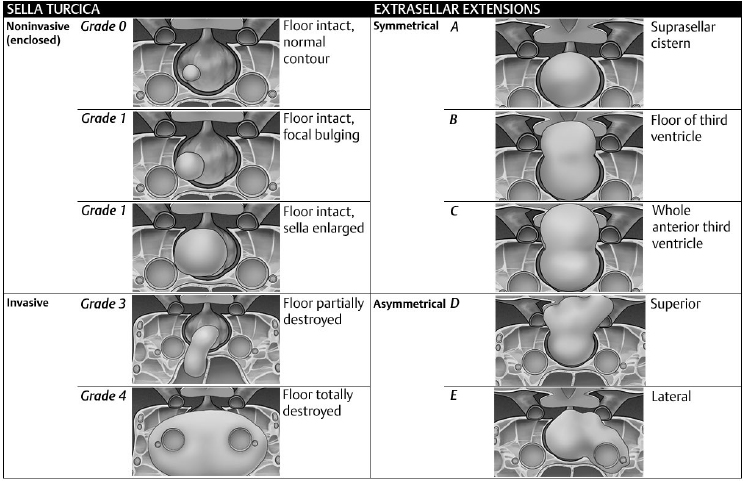
 Try to save a sufficient amount of tumor for pathology analysis (including electron microscopy). In cases of a very soft or very small tumor, which can be easily aspirated, a “suction trap” connected to the surgical suction tubing may be useful.
Try to save a sufficient amount of tumor for pathology analysis (including electron microscopy). In cases of a very soft or very small tumor, which can be easily aspirated, a “suction trap” connected to the surgical suction tubing may be useful.