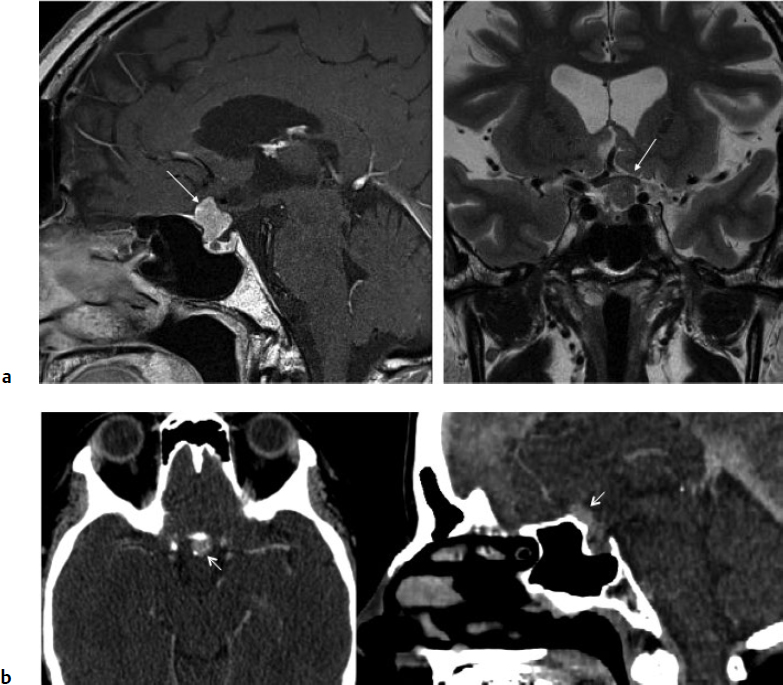
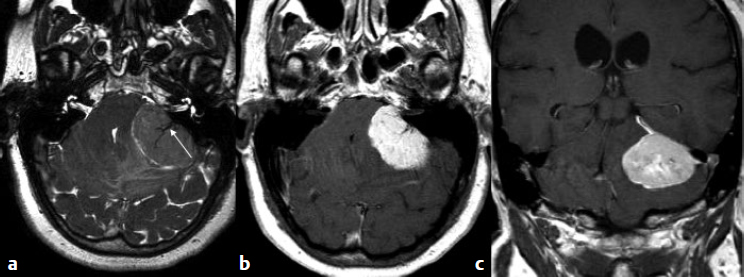
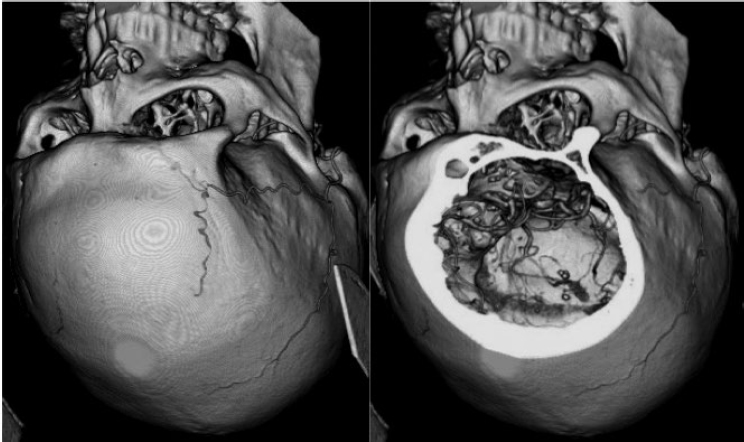
5 Skull Base Imaging • The radiologist is an active member of the skull base team and has a very important role in the outcome of the patient. • Surgeons should treat every request they make for imaging in the same way as making a request for a consultation from a clinical specialist. Requests and responses should be tailored to the patient’s symptoms and signs, and should provide the radiologist with clinical information that will aid in diagnosis. This is critical for optimum patient care (Fig. 5.1). Radiology Pearl Good communication with the radiologist is essential to good outcomes. Final diagnosis comes from the pathologist. However, a radiologist’s opinion regarding diagnosis is extremely helpful to the team making management decisions. A purely descriptive report that skirts or avoids a working diagnosis is of much less value. Information regarding the location of the tumor should also specify the origin and extent of the tumor and relationships to critical structures. • Determining the origin can provide a clue to pathology (Fig. 5.2). The origin can be ascertained in many ways: by the epicenter of the lesion, by focal hyperostosis or erosion in certain lesions, and by the way the surrounding structures are displaced. Fig. 5.1a,b (a) A tuberculum sellae meningioma (arrow) elevating the chiasm is readily apparent on the appropriate magnetic resonance imaging (MRI) sequences: sagittal postgadolinium T1 (left), coronal T2 (right). Note an incidental microadenoma within the pituitary gland (arrow). (b) A small tuberculum sellae meningioma, however, may be difficult to diagnose on axial computed tomography (CT) unless the appropriate technique is used (thin axial sections and a sagittal reformat in this example). The radiologist cannot plan the appropriate CT technique or MRI protocol without adequate clinical information on the requisition. • Extent: Information about patterns of growth is important in understanding pathology (Figs. 5.3 and 5.4). Extension into foramina and fissures, across bone and dura, in proximity to and with encasement of vessels and nerves can alter management and help determine the surgical plan.1 • Understanding the extent also helps to determine the stage and volume of the tumor. The determination of volume and any change in volume, signifying growth, is critical in deciding on the management of benign tumors such as meningiomas. Fig. 5.2a–c A cerebellopontine angle meningioma on axial T2 (a), postgadolinium T1 axial (b), and coronal (c) views. The eccentric relationship to the internal auditory canal (IAC), the broad base on the dura, an obtuse angle of contact, and the enhancing dural tail are obvious clues to the diagnosis. In addition, a converging leash of vessels (a, arrow) and focal hyperostosis (if present) can provide additional clues to the exact site of origin. • Relationships: Three-dimensional (3D) rendering can help in understanding in detail the relationships among bone, soft tissue, and vessels. Because 3D images can be rotated, sectioned, and viewed at any desired angle to simulate the surgical approach, they can help guide management (Fig. 5.5). Fig. 5.3 Facial schwannoma presenting as an enhancing extra-axial mass in the middle fossa. Such a lesion can be mistaken for a meningioma; enhancement of the facial nerve is the clue to the diagnosis (arrow). Careful inspection of all possible pathways of spread is an essential step in skull base imaging. Note fat suppression used on these postgadolinium T1 images. By understanding the location and extent of the tumor, an understanding of resectability and the risks of resection are possible. Thus, with a proper and careful diagnosis, it is useful to determine which treatment approach—observation, surgery, radiation, or palliation—can achieve the goals desired for the patient. Proper diagnosis can help to plan the surgical approach or the radiation plan, determine the prognosis, and decide on a follow-up schedule.2 Good-quality imaging data transferred to the operating room are essential and routinely used for safe surgical navigation.3 The surgeon must understand not only the location of the tumor and the pathology but also where normal structures are expected to be found. For example, does the meningioma originate medial to or lateral to the entrance of cranial nerve (CN) V into Meckel’s cave? If medial, CN V will be on the superficial side of the tumor when accessed by a retrosigmoid or petrosal approach, meaning that the surgeon will encounter the trigeminal nerve at the beginning of the tumor resection. If the origin is lateral, then the surgeon accessing the tumor from any posterolateral approach will encounter the nerve at the end of the tumor resection, preferably deep to the arachnoid of the trigeminal cistern. The same principles can be enunciated for the arteries and veins around the tumor and along the pathway of access. Understanding these relationships is important for optimal surgical outcomes. Understanding these navigational issues requires close communication between radiologists and surgeons. Preoperative embolization is the most common adjunctive treatment. Intra-arterial chemotherapy and procedures to open the blood–brain barrier are more recent additions to the role of interventional neuroradiologists (see Chapter 12).4 The complementary role of computed tomography (CT) and magnetic resonance imaging (MRI) is long known and well established, but cannot be overemphasized.5 Each technique has advantages, but most skull base pathology requires both modalities for a complete evaluation. • Computed tomography is the obvious choice in evaluating the bony skull base (bone tumors, erosion of bone by soft tissue tumors and infection, fractures, cerebrospinal fluid [CSF] leaks). • It provides exquisite bony detail, high spatial resolution vascular imaging, and excellent 3D images, and enables detection of calcium and acute blood. • It is superior in detecting subtle erosions of cortical margins and foramina, and in demonstrating reactive changes in bone (e.g., hyperostosis and pneumosinus dilatans around meningiomas) or tiny areas of calcification within lesions.5,6 • A thin-section axial helical CT data set is initially acquired usually with contrast; 64-slice scanners are standard in most skull base centers, but some centers have access to 256-slice or higher scanners with better computed tomography angiography (CTA) and 3D reconstructions, although there is no clear clinical advantage in the skull base.7 • Axial images of desired thickness are then generated, and high-quality coronal and sagittal images are reconstructed, using soft tissue and bone algorithms. • It is important to use the correct “bone algorithm” images, which are generated from the same data set with edge enhancement to evaluate bone in sharp detail. Looking at the routine soft tissue images using a bone window setting is suboptimal. • Intravenous contrast is necessary to look for intracranial or extracranial disease, to evaluate the vessels and cavernous sinus, and to help characterize the lesion. Contrast provides an initial assessment of tumor vascularity and may determine the need for dedicated vascular imaging [CTA/magnetic resonance angiography (MRA) or digital subtraction angiography (DSA)]. CT and MR perfusion studies have been used for grading the tumor, determining the prognosis, assessing the treatment response, and differentiating treatment/radiation effects and nonneoplastic lesions from neoplasms.8 • Contrast may be omitted on follow-up CT of osseous tumors with no soft tissue components, of sinonasal lesions clearly without intracranial extension, or if there is a contraindication. • An unenhanced CT should be performed first if intracranial hemorrhage (or calcification) is a concern; contrast images can follow. • Patients known to be at higher risk for contrast allergy may be premedicated using the following regimen (all three medications) to reduce the frequency or severity of reactions, after obtaining informed consent9: • Computed tomography angiography (CTA) is invaluable in preoperative planning, particularly when intracranial disease is in close proximity to arteries, the cavernous sinus, or the dural sinuses. It is also important to recognize variant vascular anatomy such as a persistent trigeminal artery or unexpected vascular lesions such as aneurysms.10 • Three-dimensional images can be postprocessed from the CTA source data set (Fig. 5.5). • A “less is more” approach is advocated when ordering CTA studies. A focused region of interest (ROI) should be clearly defined in the radiology request. • Coverage beyond the ROI results in unnecessary radiation, redundant images, and lower quality images at the region of interest. One study quotes an effective dose of 0.67 millisievert (mSv) for CTA of the cerebral vessels and 4.85 mSv for the cervicocerebral vessels.11 No radiation risk is acceptable, however small, without benefit to the patient.12 As an example, a CTA covering from C2 to the circle of Willis will provide high-quality vascular imaging at the skull base. This advantage is lost on a CTA covering the aortic arch to the vertex. • Computed tomography angiography will show vascular variants of surgical importance10,13: • Computed tomography venography is done by acquiring a similar axial data set following intravenous contrast, but later in the venous phase. • Performing both CTA and CTV involves twice the amount of radiation.11 The second acquisition may be difficult to achieve, resulting in suboptimal CTV images, particularly in patients with cardiac dysfunction. • Cisternography is occasionally indicated for detection or confirmation of the site of a CSF leak along the skull base. It is usually performed if unenhanced high-resolution CT of the skull base and/or MR cisternography fail to show the site of CSF leakage.14,15 • Ideally, to have maximum sensitivity, the patient should be actively leaking at the time of the test. • The procedure involves intrathecal administration of nonionic iodinated myelographic contrast medium under fluoroscopy, usually by lumbar puncture, occasionally by lateral C1-C2 puncture. The usual adult doses are 10 to 15 mL of nonionic contrast at concentrations not exceeding 300 mg/mL, and up to a maximum dose of 3 g. Strict adherence to safe practice guidelines is mandatory, as inadvertent use of ionic contrast can cause seizures and death.16 • The contrast column is gravitated cranially by tilting the fluoroscopy table, followed by high-resolution CT of the skull base with coronal reconstructions. Direct coronal CT with the patient in the prone position may be attempted in CSF rhinorrhea. • Also see Magnetic Resonance Cisternography, below. • Magnetic resonance imaging is clearly superior in evaluating the soft tissues, particularly neoplastic intracranial extension, dural integrity, leptomeningeal spread, and brain invasion.17,18 • The same applies to perineural spread, orbital extension, cavernous sinus involvement, and extension into fissures and foramina.19 • Routine brain sequences are insufficient; dedicated skull base protocols are required. These commonly include T1, fat-suppressed T2, or short tau inversion recovery (STIR) in appropriate planes, focused on the ROI and not the whole brain (Tables 5.1 and 5.2). • Specifying anterior, middle, or posterior skull base, and specifying regions of interest such as orbital apex, cavernous sinus, cerebellopontine angle (CPA), sella, clivus, foramen magnum, etc., will prompt a protocol dedicated to that region by the radiologist. Without this information, studies cannot be tailored to the ROI (Fig. 5.1). • Gadolinium is required for the detection of intracranial disease, cavernous sinus involvement, and meningeal and perineural spread, usually with fat suppression. Documenting enhanced scans in the three primary orthogonal planes is very helpful in tumor surgery planning. • Bright signal from fat and marrow may mask a lesion or make enhancement unappreciable. Fat-suppression techniques (Figs. 5.3 and 5.6) are therefore invaluable, but can be a double-edged sword, as they are prone to susceptibility artifacts. Artifacts from dental hardware, clips, and implants are markedly exaggerated on fat-suppressed sequences. Table 5.1 Common Magnetic Resonance Imaging (MRI) Sequences Used in the Skull Base
 Interaction of the Radiologist and Surgeon
Interaction of the Radiologist and Surgeon
 Role of Neuroimaging in Skull Base Surgery
Role of Neuroimaging in Skull Base Surgery
Diagnosis
Location
Resectability and Risk Assessment
Surgical Navigation
Adjunctive Treatment
 Imaging Choices
Imaging Choices
Computed Tomography
 Prednisone 50 mg PO at 13 hours, 7 hours, and 1 hour before
Prednisone 50 mg PO at 13 hours, 7 hours, and 1 hour before
 Diphenhydramine PO 50 mg at 13 hours, 7 hours, and 1 hour before
Diphenhydramine PO 50 mg at 13 hours, 7 hours, and 1 hour before
 Ranitidine PO at 1 hour before contrast administration
Ranitidine PO at 1 hour before contrast administration
Computed Tomography Angiography
 The internal carotid artery (ICA) medially positioned, bulging into sphenoid sinus. The bony wall may be thin or deficient, putting the ICA at risk during transsphenoidal surgery. Manipulation of a septum attached to this thin wall may also jeopardize the ICA.
The internal carotid artery (ICA) medially positioned, bulging into sphenoid sinus. The bony wall may be thin or deficient, putting the ICA at risk during transsphenoidal surgery. Manipulation of a septum attached to this thin wall may also jeopardize the ICA.
 A persistent trigeminal artery may also bulge into the sphenoid sinus.
A persistent trigeminal artery may also bulge into the sphenoid sinus.
 A persistent hypoglossal artery traverses the hypoglossal canal (which may be enlarged). This rare variant may be the major or sole contributor to the basilar system. Another rare variant is the proatlantal artery, an ICA or external carotid artery (ECA) branch that supplies the vertebral artery.
A persistent hypoglossal artery traverses the hypoglossal canal (which may be enlarged). This rare variant may be the major or sole contributor to the basilar system. Another rare variant is the proatlantal artery, an ICA or external carotid artery (ECA) branch that supplies the vertebral artery.
 A high-riding jugular bulb may be at risk during translabyrinthine surgery; often visible on coronal CTA images; best seen on computed tomography venography (CTV).
A high-riding jugular bulb may be at risk during translabyrinthine surgery; often visible on coronal CTA images; best seen on computed tomography venography (CTV).
 Venous varix at the basiocciput can occasionally be mistaken for a bony lesion.
Venous varix at the basiocciput can occasionally be mistaken for a bony lesion.
Computed Tomography Venography
Cisternography
Magnetic Resonance Imaging
MRI Sequence | Appearance/Role |
T1 | • Fat is T1 bright (marrow, extracranial fat, dermoid, lipoma). Displacement of fat planes can help detect skull base lesions (“fat is a friend”). Replacement of marrow fat can help detect bone involvement. • Methemoglobin (subacute hematoma), melanin, and highly proteinaceous fluids (e.g., Rathke’s) are also T1 bright. • Water and cerebrospinal fluid (CSF) are T1 dark. Most pathologies are relatively dark on T1 unless fatty, hemorrhagic, or proteinaceous. |
T2 | • Water, CSF, cystic and necrotic areas, fluid collections, and edema are T2 bright. Most tumors are bright and stand out on T2, but not all tumors. • Tumors with tightly packed cells and high nuclear cytoplasmic ratio are relatively T2 dark (lymphoma, primitive neuroectodermal tumor, germinoma), and so are meningiomas (variable). Calcium and hemosiderin are dark on T2. Fungal hyphae are very dark on T2. Flowing blood causes signal dropout (flow voids) often better seen on T2 |
Fluid-attenuated inversion recovery (FLAIR) | • Useful for evaluating effect on the adjacent brain (edema, gliosis). Epidermoids appear bright on FLAIR and stand out against dark CSF signal |
Diffusion-weighted imaging (DWI) | • Acute infarction, abscess, and epidermoids show restricted diffusion (bright on DWI, dark on apparent diffusion coefficient [ADC]). Make sure it is not T2 shine-through (bright on both). |
Fat suppression (FS), FS T2/T2 short-tau inversion recovery (STIR), FS T1 postgadolinium | • Fat signal can mask bright pathology on T2. • Fat is very bright on T1, and can mask enhancement. • Suppression of fat signal in marrow, subcranial soft tissues, orbit and craniovertebral junction (for ligamentous injury) is therefore invaluable in skull base imaging. |
Radiology Pearl
Fat suppression techniques are prone to artifacts and are highly sensitive to motion; inhomogeneous fat suppression can make interpretation difficult.
Table 5.2 Magnetic Resonance Imaging Appearance of Intracranial Hematoma
Hyperacute blood | Isointense to dark on T1 | Bright on T2 |
Acute hematoma | Dark on T1 | Dark on T2 |
Early subacute | Bright on T1 | Dark on T2 |
Late subacute | Bright on T1 | Bright on T2 |
Chronic | Dark on T1 | Dark on T2 (from periphery to center) |
• Fat-suppressed and non–fat-suppressed images may be complementary, but acquiring them will take double the time. Every choice has a trade-off in MRI. MR sequences and planes of section therefore need to be carefully chosen.
Radiology Pearl
A “one-size-fits-all” approach does not apply.
• The protocol needs to be tailored so that the best planes of section and the most appropriate sequences are applied, every time. As an example, a clival lesion may be best seen on a midsagittal thin-section T2 sequence, but this may not be part of the “routine” protocol.
• Once established, the same protocol should be used on follow-up imaging to facilitate comparisons. This may be requested on follow-up requisitions.
• “Less is more” makes for better MR image quality. A smaller field of view (more focused imaging) translates into higher resolution images in the same amount of time. A rough sketch on the requisition can be very effective.
Radiology Pearl
Ordering too many scans during one sitting may be counterproductive.
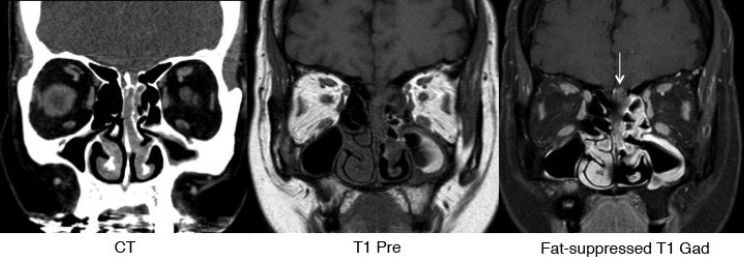
Fig. 5.6 Sinonasal mass (esthesioneuroblastoma) with intracranial extension that is best identified on the fat-suppressed postgadolinium coronal T1 image on right (arrow). Fat-suppressed sequences are invaluable in the skull base and orbit.
• Patients tolerate shorter scans better. Long studies are often degraded by patient motion.
Magnetic Resonance Cisternography
This is performed using highly T2-weighted 3D thin-section “myelographic” sequences. These can be reconstructed in multiple planes. Intrathecal injection of gadolinium is also an option, but is not widely used as of yet.20
Cerebrospinal Fluid Flow Studies
Cerebrospinal fluid flow studies traditionally use the phase contrast technique; newer techniques, which use variations of arterial spin labeling, are now available.21 These techniques can demonstrate the presence or absence of CSF flow across the foramen magnum in a Chiari malformation, across the aqueduct of Sylvius, within an obstructed ventricular system (such as posterior fossa tumor), across a third-ventriculocisternostomy, and across a fenestrated arachnoid cyst. A series of images are acquired over short periods of time, which are then viewed as a cine sequence.
Magnetic Resonance Tractography
Advanced diffusion tensor imaging methods on 3-tesla or higher systems can map the facial nerve preoperatively in patients with large CPA tumors.22 Similarly, preoperative mapping of CNs II, III, V, and VIII has been achieved and its clinical utility is being examined.23
Magnetic Resonance Angiography
• Magnetic resonance angiography is usually performed using gadolinium. If vascular imaging is not a high priority and gadolinium is not otherwise indicated, or the patient has an allergy to gadolinium, time of flight (TOF) MRA may be considered.
• If a CTA has already been performed, MRA is usually unnecessary. Exceptions include suspected dissection at the skull base with a negative CTA, or the presence of metal artifact on the CTA from coils.
Magnetic Resonance Venography
• Magnetic resonance venography (MRV) is best achieved using gadolinium, as TOF MRV is prone to artifacts. A common indication is to look for encroachment of a dural sinus by a skull base meningioma.
Digital Subtraction Angiography
• The development of high-quality cross-sectional vascular imaging (CTA, MRA) has mostly obviated the need for DSA using catheter techniques, except as part of interventional procedures. Additional indications are as follows:
 Patients with severe artifacts from implants on CTA and MRA, or artifacts on CTA with a contraindication to MR; for example, a patient with an aneurysm clip that is unsafe for MRA and obscures the area of interest on CTA will need a DSA.
Patients with severe artifacts from implants on CTA and MRA, or artifacts on CTA with a contraindication to MR; for example, a patient with an aneurysm clip that is unsafe for MRA and obscures the area of interest on CTA will need a DSA.
 Digital subtraction angiography remains the gold standard and serves as a problem-solving tool when findings are not fully clarified on CTA or MRA (such as suspicion of subtle vascular injury or confirming patency of a small encased branch).
Digital subtraction angiography remains the gold standard and serves as a problem-solving tool when findings are not fully clarified on CTA or MRA (such as suspicion of subtle vascular injury or confirming patency of a small encased branch).
 Occasionally, a patient with renal failure can undergo a limited DSA using less contrast than a CTA requires.
Occasionally, a patient with renal failure can undergo a limited DSA using less contrast than a CTA requires.
• Digital subtraction angiography can demonstrate vascular displacement, stretching, encasement, stenosis, occlusion, collaterals, tumor vascularity, angiogenesis, and vascular injury.
• It assesses the lumen only and cannot demonstrate extraluminal pathology the way MR or CT can (except indirectly by studying the effect on the lumen and tumor vascularity).
• Digital subtraction angiography, on the other hand, provides temporal resolution with arterial, capillary and venous phase images not available on MRA or CTA. DSA is therefore indicated for evaluating arteriovenous shunting lesions, such as dural arteriovenous fistula (DAVF) and arteriovenous malformation (AVM); for determining the timing and adequacy of collateral flow when an artery is stenosed or occluded; and for assessing venous drainage when a dural sinus is compromised, such as by a meningioma.
• Time-resolved MRA techniques [such as Time-Resolved Imaging of Contrast KineticS (TRICKS), 4D Time-Resolved Angiography using Keyhole (4D TRAK), and Time-resolved angiography WIth Stochastic Trajectories (TWIST)] are a promising tool that can occasionally obviate the need for a catheter angiogram, but they cannot match the spatial or the temporal resolution of conventional angiography as of yet.24,25
• Neurologic complications occur in 1.3% of catheter angiographies, of which some are transient or reversible, but 0.5% of deficits are permanent.26
• Additional complications include hematoma around the puncture site (4.2%), nausea/vomiting and transient hypotension (1.2%), and headache, chest pain, arrhythmia, urticaria, anaphylaxis, circulatory failure, and acute renal failure (each below 1%). Risk of death from all complications is 0.06%, mostly related to neurologic complications.27
• Relative contraindications to catheter cerebral angiography include hypotension, coagulopathy, clinically significant sensitivity to contrast material, renal insufficiency, and congestive heart failure. The patient can be optimized prior to angiography (for example, with premedication for contrast allergy), provided that the benefits outweigh the risks. Planning and prior discussion with the radiologist is essential.28
Nuclear Medicine
• Thallium-201 and technetium-99m (Tc-99m) glucoheptonate in conventional nuclear imaging, as well as sugar analogues such as fluorodeoxyglucose (FDG), radiolabeled amino acids, and cell membrane analogues in positron emission tomography (PET) are available to distinguish viable tumor from edema and necrosis in the skull base.29,30
• Osteomyelitis imaging in the skull base is best performed with tagged white blood cells, which is commonly achieved with either a Tc-99m or indium-111 (In-111) label rather than gallium-67, as the latter often fails to accumulate in marrow-rich bone such as skull.31–33
• Shunt studies are frequently performed via injection of 0.1 to 0.5 cc of pyrogen-free Tc-99m diethylenetriamine pentaacetic acid (DTPA) into the reservoir using sterile technique.34 Delay in tracer migration beyond an hour with ambulation and shunt pumping is highly suspicious for obstruction.
• Tumors of the skull base including meningiomas and pituitary adenomas can be assessed by both somatostatin analogues such as In-111 octreotide and norepinephrine analogues such as iodinated meta-iodo-benzyl-guanosine.35–38
• Phosphorus-32 is an electron emitter with a 14.3-day half-life, usually prepared as a colloid, that has gained acceptance for the control of craniopharyngioma usually via direct transnasal injection, with some authors reporting a 70% tumor control rate at 10 years.39–42
Plain X-Rays
• Plain radiography has a very limited role in modern skull base imaging, except in the initial evaluation of the craniovertebral junction anomalies, basilar invagination, and ligamentous instability.43
• Lines and measurements can be used in the assessment of basilar invagination (digastric line and bimastoid line on the anteroposterior radiograph, and McRae, Chamberlain, and McGregor lines on the lateral radiograph).44 CT or MRI can provide the same information and also demonstrate any mass effect on neural structures.45
• Useful information may still be obtained from plain X-rays in a low-resource environment. Lytic or sclerotic bone involvement, focal hyperostosis, enlargement of skull base foramina, vascular grooves (especially in the presence of occluded venous sinuses), opacification, enlargement or destruction of a paranasal sinus, displacement of soft tissue planes, and soft tissue calcifications can provide evidence of an underlying lesion.46
 Radiology of Selected Skull Base Pathologies
Radiology of Selected Skull Base Pathologies
See boundaries of the skull base fossae in Chapter 2, Fig. 2.3, and in Fig. 5.7. Tables 5.3 and 5.4 summarize the imaging choice criteria and main radiological findings of skull base pathologies. Table 5.5 summarizes anterior skull base (ASB) pathologies.
Meningioma
Radiology Pearl
In most instances, specifying anterior, middle, or posterior skull base location on the requisition is sufficient. A diagram on the requisition can be invaluable to the radiologist for planning the appropriate protocol.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree