Treatment
% Sensitized
% Nonsensitized
VEH+COC
49.02
50.98
7-NI+COC
31.04*
68.96
ODQ+COC
27.78**
72.22
SILD+COC
67.44***
32.56
The NAS works as an interface between limbic and motor systems [102] and drug seeking behavior depends on glutamate transmission in this structure [103]. HP is one of the glutamate projecting afferents to NAS [104], and synaptic changes occurring in this structure can affect the level of neuronal activity within the NAS. HP LTP has long been postulated to underlie learning and memory processes and may play a role in the complex associative learning that contributes to drug-seeking behavior and relapse [42]. In fact, repeated COC administration enhances HP LTP [105], and theta burst stimulation of HP ventral subiculum reinstates COC-seeking behavior in rats [52]. We reported that COC sensitization induced an increased HP synaptic transmission, observed as a reduction in the threshold to generate LTP [50], and this enhancement was prevented by nNOS inhibition. Similar results were observed when sGC was inhibited by ODQ administration during repeated COC. On the other hand, when cGMP availability was increased using SILD, the COC-facilitated synaptic transmission was maintained [74]. This facilitation could indicate an increased susceptibility to stimuli able to strength glutamate synapses. Then, activation of nNOS/NO/sGC/cGMP pathway by repeated COC may facilitate the LTP generation in HP, activating glutamate containing pathways to NAS, finally responsible for motor execution of goal-directed behavior such as sensitization. These results are in accordance with the effects of cGMP up regulation on cortico-striatal synaptic transmission in vivo [106]. Surprisingly we further observed that the acute or repeated SILD treatment facilitated synaptic transmission in HP dentate gyrus, indicating a possible enhancement in the strength of glutamate synapses by sildenafil exposure [74], agreeing with previous reports showing that SILD induces long-term memory retention and reconsolidation [107] and rescues synaptic plasticity in an Alzheimer disease mouse model [108].
It has been demonstrated that a single COC exposure increases NO release in mPFC, HP, and striatum [109, 110] and repeated administration enhances nNOS activity and NO production in many brain regions [111]. We have observed that nNOS activity was increased in HP only in sensitized animals (VEH+COC-S) (Fig. 13.1). Furthermore, the percentage of nNOS activity increase in this group was considerably greater than in the acute COC group. These results may indicate that nNOS enzyme evidenced a neuroadaptative process of “sensitization” after repeated administration, which is associated with behavioral sensitization and elevated efficiency of hippocampal synaptic transmission [50]. We also showed that inhibition of nNOS during repeated COC administration (7-NI+COC-S) prevented the “sensitization” of the enzyme activity observed in sensitized animals (VEH+COC-S) (Fig. 13.1). These results are further supported by the correlation analysis between locomotor activity on day 5 and NO levels in both sensitized and nonsensitized groups in which high locomotor activity is positively correlated with elevated NO levels. However, correlation was weakened when nNOS inhibitor was administered concomitantly to COC (Fig. 13.2) [74]. Systemic administration of different nNOS/NO/sGC/cGMP modulators such as 7-NI, ODQ, or SILD, affects NO or cGMP availability in the whole CNS. Thus, changes observed in the HP can be the result of a primary effect of such inhibitors in brain areas related to the reward circuit that project to the HP, such as the VTA, modulating neuronal activity. A local effect in the HP cannot be ruled out because single or repeated COC administration induces increments in nNOS activity in this area.
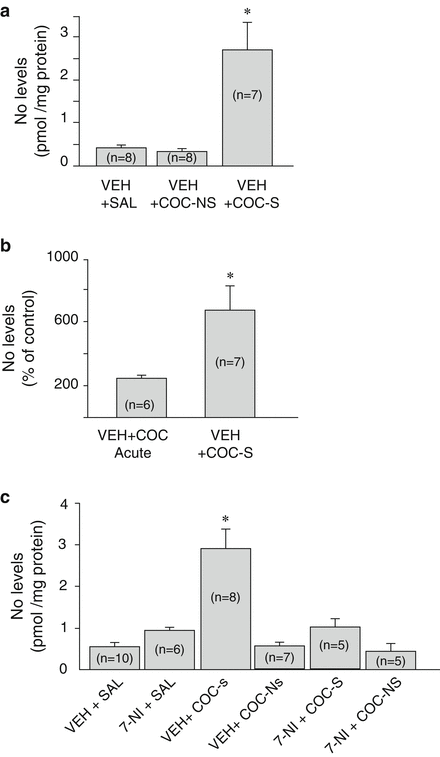
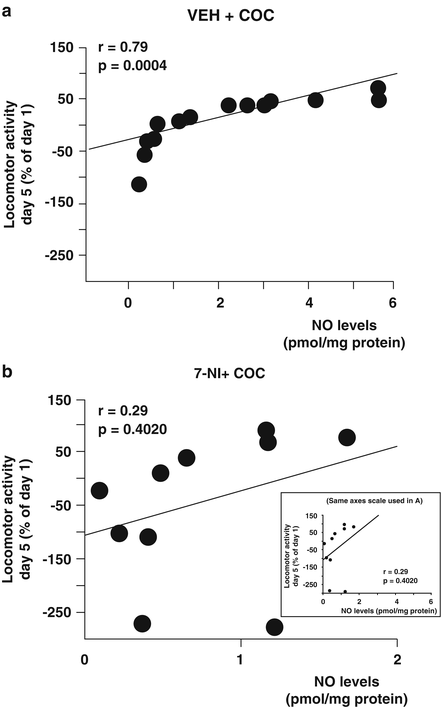

Fig. 13.1
Cocaine sensitization increases neuronal nitric oxide synthase (nNOS) activity in the hippocampus. Bars graph indicating nitric oxide (NO) levels in: (a) sensitized and nonsensitized groups with or without nNOS inhibition and their controls, *p < 0.05 compared with other groups. Results are expressed as means of NO pmol/mg protein ± standard error (S.E.); (b) acute treated animals [vehicle (VEH)+cocaine (COC) Acute] and sensitized rats [VEH+cocaine-sensitized (COC-S)], *p < 0.05. Results are expressed as means of percentage of their respective controls [VEH+saline (SAL) acute and VEH+SAL] ± S.E. Number of animals are indicated in parenthesis (adapted with permission from Springer Science+Business Media: Psychopharmacology, Involvement of nNOS/NO/sGC/cGMP signaling pathway in cocaine sensitization and in the associated hippocampal alterations: does phosphodiesterase 5 inhibition help to drug vulnerability? Fig. 3 of [74])

Fig. 13.2
Development of sensitization correlates with high levels of nitric oxide production in the hippocampus. Graphs showing correlation between the percent of increase in locomotor activity (day 5 respect to day 1) and the threshold to generate long-term potentiation (LTP) in: (a) vehicle (VEH)+cocaine (COC) (sensitized and nonsensitized) treated rats and (b) 7-nitroindazole (7-NI)+COC (sensitized and nonsensitized) treated rats. The correlation coefficients (r) are indicated in bold (adapted with permission from Springer Science+Business Media: Psychopharmacology, Involvement of nNOS/NO/sGC/cGMP signaling pathway in cocaine sensitization and in the associated hippocampal alterations: does phosphodiesterase 5 inhibition help to drug vulnerability? Fig. 4 of [74])
Reversion of Diazepam Dependence: Behavioral and Pharmacological Strategies
It has been demonstrated that the environmental context of an experience induced by a drugs of abuse is crucial for drug-seeking behavior and relapse in human addicts [64]. Furthermore, learning and memory, particularly contextual memories, are important for the establishment of conditioned responses to drugs of abuse [64, 65]. In our work we described that environmental cues related to drug experience have a direct impact on retrieval of the memory acquired when the DZ withdrawal signs were associated with the context of withdrawal because alteration in the cues presented 24 h after last administration prevented the expression of the anxiety-like behavior when animals were reexposed, 15 days after last administration, to the withdrawal environment. Alterations of contextual cues consisted of a new operator, modified location of the animals in the plus maze test room from the original assessment, and the animals were held with a piece of fabric. These changes constitute a significant alteration in the context of withdrawal and are able to modify conditioned responses previously observed. It is interesting to point out that manipulation of environmental cues during reexposure prevented the expression of anxiety-like behavior in the majority of animals considered to be DZ dependent. Only a small portion of them expressed anxiety during reexposure to the withdrawal environment in spite of the altered contextual cues [112]. This differential susceptibility could be attributed to the fact that the associative component can be particularly strengthened in these animals, and the environmental changes are not enough to disrupt the acquired memory. Moreover, because of the experimental design in which the experimental groups were defined by classification, we cannot rule out the possibility that other selected characteristics, unrelated to anxiety or dependence, may have caused the observed effects.
We have recently demonstrated that DZ dependence is associated with an increased HP synaptic plasticity in both short- [57] and long-term withdrawal [58]. Furthermore, alteration in contextual cues associated with the withdrawal experience previously described, not only impaired the expression of the anxiety-like behavior, but also disrupted the facilitated HP LTP in most of the DZ-dependent animals reexposed to the altered withdrawal environment. Interestingly, the animals that expressed anxiety-like behavior in spite of the altered contextual cues preserved the facilitation in HP LTP [112]. These persistent changes in synaptic strength during synaptic plasticity require new gene expression and protein synthesis [58, 113, 114]. The immediate-early gene activity-regulated cytoskeleton-associated protein (Arc) has been proposed as a marker of neuronal activity linked to synaptic plasticity and to consolidation of spatial memory in the HP, acquiring a key role in the organization of information storage in the CNS [115, 116]. We have reported increased HP Arc protein levels related to the facilitated LTP in DZ-dependent animals during short-term withdrawal [58]. Similar to the observed electrophysiological changes, we observed that the increased HP Arc protein levels were reversed in DZ-dependent animals reexposed to the plus maze test with contextual cue changes. However, only the small proportion of animals that expressed anxiety in spite of the altered contextual cues maintained increased Arc protein levels, indicating that Arc could be responsible, at least in part, for the maintenance of the facilitated HP LTP during this period of withdrawal. These results further support the hypothesis that the HP is a brain structure involved in the associative memory formation and retrieval when contextual cues associated with drug exposure and withdrawal are presented to a dependent subject [58]. Moreover, it has been suggested that Arc promotes LTP consolidation through regulation of actin dynamics [117]. In addition to the maintenance of synaptic potentiation during late-LTP, long-term memories are dependent on the persistent phosphorylation and sustained activation of the brain-specific atypical protein kinase M zeta (PKMζ) [118]. It is also known that actin polymerization is critical for PKMζ synthesis, leading authors to suggest the possibility of a sequential mechanism of LTP maintenance in which Arc-dependent stabilization of F-actin promotes PKMζ synthesis and expression of enduring LTP [117]. We have described that inhibition of PKMζ using the zeta pseudosubstrate inhibitory peptide (ZIP, selective PKMζ inhibitor) prior to reexposure to the withdrawal environment with preserved contextual cues prevented the anxiety expression. Furthermore, LTP facilitation in all DZ-dependent animals was reversed, suggesting that plasticity in the HP has a crucial role in maintenance of these memories that were vulnerable to the amnesic effects of ZIP [112, 118]. These results are in accordance with the hypothesis that the persistently active PKMζ might perpetuate information both at synapses and during long-term memory [119]. Furthermore, LTP was generated in DZ-dependent animals intra-HP infused with ZIP at a similar frequency to control animals, whereas vehicle infusion in DZ-dependent animals did not affect the lower LTP threshold associated with exposure to the contextual cues related to DZ withdrawal experience as previously described. Therefore, inhibition of PKMζ in long-term withdrawal reversed the facilitated LTP generated during DZ chronic administration and maintained through withdrawal, without affecting the mechanisms involved in vitro LTP generation [112].
Final Remarks
Abuse of psychostimulants such as cocaine and amphetamines, and misuse of prescribed drugs with or without a prescription represent a significant health and social problem worldwide. A major problem in the treatment of drug addiction is relapse to drug abuse. Then, highlighting common mechanisms underlying persistence of abuse to different drugs could be interesting for the development of strategies of effective treatments. Our work is focused on the study of common mechanisms underlying COC sensitization, as a model of craving and relapse, and DZ dependence. We hypothesize that up regulation of NOS/NO/sGC/cGMP signaling pathway in different brain areas could initiate, contribute, or exacerbate addictive behaviors in humans. These results are of significant importance considering that patients participating in drug detoxification programs or addicts in withdrawal may use other drugs to mitigate withdrawal symptoms or side effects of the primary drug used. In fact, the misuse and recreational use of PDE5i have been described in different human populations [120] and linked to the illicit use of drugs of choice for abuse [121]. Taking our results into consideration, we can speculate that PDE5i may increase vulnerability to drug abuse.
Moreover, our results demonstrate that memories related to drug experience and withdrawal are relevant to the expression of anxiety-like behavior when DZ-dependent animals are reexposed to the contextual cues associated with the withdrawal experience. We further characterize the HP as a pivotal brain structure involved in engagement of learning processes related to drug dependence and withdrawal. These findings contribute to the establishment of the mechanisms by which drugs of choice for abuse can alter brain function that can be used as treatment or prevention of drug dependence and abuse.
Disclosures/Conflicts
None.
References
1.
Luddens H, Korpi ER, Seeburg PH. GABAA/benzodiazepine receptor heterogeneity: neurophysiological implications. Neuropharmacology. 1995;34(3):245–54.PubMed
2.
Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21(3):189–274.PubMed
3.
Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47(2):181–234.PubMed
4.
Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16(5):162–8.PubMed
5.
Chaudieu I, St-Pierre JA, Quirion R, Boksa P. GABAA receptor-mediated inhibition of N-methyl-D-aspartate-evoked [3H]dopamine release from mesencephalic cell cultures. Eur J Pharmacol. 1994;264(3):361–9.PubMed
6.
Stelzer A, Slater NT, ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326(6114):698–701.PubMed
7.
Corda MG, Orlandi M, Lecca D, Giorgi O. Decrease in GABAergic function induced by pentylenetetrazol kindling in rats: antagonism by MK-801. J Pharmacol Exp Ther. 1992;262(2):792–800.PubMed
8.
Giorgi O, Orlandi M, Geic M, Corda MG. MK-801 prevents the decrease in 35S-TBPS binding in the rat cerebral cortex induced by pentylenetetrazol kindling. Brain Res Bull. 1991;27(6):835–7.PubMed
9.
Ohkuma S, Chen SH, Katsura M, Chen DZ, Kuriyama K. Muscimol prevents neuronal injury induced by NMDA. Jpn J Pharmacol. 1994;64(2):125–8.PubMed
10.
Tsuda M, Suzuki T, Misawa M. Recovery of decreased seizure threshold for pentylenetetrazole during diazepam withdrawal by NMDA receptor antagonists. Eur J Pharmacol. 1997;324(1):63–6.PubMed
11.
Tsuda M, Suzuki T, Misawa M. Role of the NMDA receptor complex in DMCM-induced seizure in mice. Neuroreport. 1997;8(3):603–6.PubMed
12.
File SE. Tolerance to the behavioral actions of benzodiazepines. Neurosci Biobehav Rev. 1985;9(1):113–21.PubMed
13.
Gonzalez JP, McCulloch AJ, Nicholls PJ, Sewell RD, Tekle A. Subacute benzodiazepine treatment: observations on behavioural tolerance and withdrawal. Alcohol Alcohol. 1984;19(4):325–32.PubMed
14.
Hallstrom C, Lader M. Benzodiazepine withdrawal phenomena. Int Pharmacopsychiatry. 1981;16(4):235–44.PubMed
15.
Rickels K, Case WG, Downing RW, Winokur A. Long-term diazepam therapy and clinical outcome. JAMA. 1983;250(6):767–71.PubMed
17.
Owen RT, Tyrer P. Benzodiazepine dependence. A review of the evidence. Drugs. 1983;25(4):385–98.PubMed
18.
Petursson H, Lader MH. Benzodiazepine dependence. Br J Addict. 1981;76(2):133–45.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




