FIGURE 13.1 The layers of the retina and their relationship to the optic nerve. The inner limiting membrane is the most superficial structure; light must pass through the other layers to reach the rod and cone layer. (Modified from Ramon y Cajal S. Histologie du Système Nerveux de l’homme et Des Vertebres, vol 2. Paris, A Maloine, 1909, 1911.)
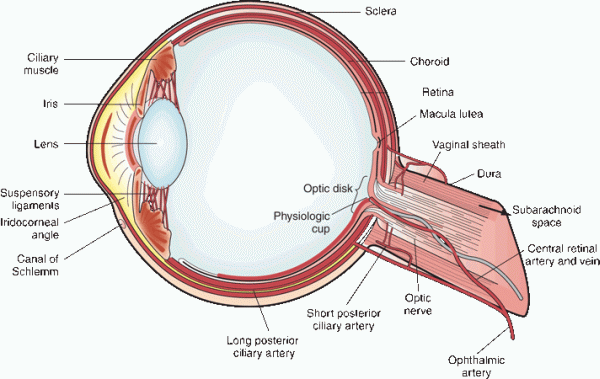
FIGURE 13.2 Structure of the eyeball.
The retinal ganglion cell axons form the retinal nerve fiber layer (NFL) as they stream toward the disc to exit through the lamina cribrosa (L. “sieve”), the collagenous support of the optic disc. Loss of axons and other abnormalities involving the NFLcan sometimes be appreciated ophthalmoscopically. Myelin in the optic nerve is CNS myelin, formed by oligodendroglia. The axons are unmyelinated in the retina and on the papillary surface, but become myelinated at the posterior end of the optic nerve head as they pass through one of 200 to 300 holes in the lamina cribrosa. In about 1% of individuals, myelin extends into the peripapillary retinal NFL(myelinated nerve fibers). Optic nerve axons primarily carry visual impulses, but they also transmit the impulses that mediate accommodation and reflex responses to light and other stimuli. Optic nerve signals are coded spatially because of the location of cells in the retina, and they are also coded temporally because the frequency and pattern of firing relays information.
Macular vision is a critical function, and the projection of the macula to the optic nerve is massive. There are approximately 1.2 million fibers in each optic nerve; about 90% arise from the macula. Because of this preponderance of macular fibers, early signs of optic nerve disease reflect macular function: impaired color vision, impaired acuity, and central scotoma. A dense collection of axons, the papillomacular bundle (PMB), travels from the nasal hemimacula to enter the temporal aspect of the disc (Figure 13.3). Fibers from the temporal hemiretina and hemimacula arch around the macula and enter the disc as the superior and inferior retinal arcades. Lesions involving these arcades may create arcuate VF defects that have an arching shape. The horizontal temporal raphe demarcates superiorly from inferiorly sweeping axons traveling from the temporal hemimacula to the disc. All of the axons from the macula gather into the PMB as it enters the optic nerve. The fibers of the PMB are very vulnerable to toxins, ischemia, and pressure.

FIGURE 13.3 The optic disc and associated structures. Axons destined to form the bulk of the fibers in the optic nerve arise from the macula, those from the nasal side form the papillomacular bundle, and those from the temporal hemimacula enter the disc as superior and inferior arcades. Spontaneous venous pulsations are best seen by looking at the tip of the column of one of the large veins on the disc surface.
The organization of the visual afferent system is not random. Tight retinotopic correlation prevails throughout the system; each point on the retina has a specific representation in the optic nerve, the chiasm, the tract, the radiations, and the cortex. The PMB, which forms the bulk of optic nerve axons, runs as a discrete bundle inside the optic nerve. The VF maintains its basic shape and structure throughout the system, although its orientation within the visual pathways changes (Figure 13.4). Fibers from the temporal hemiretina are located in the temporal half of the optic nerve, while fibers from the nasal hemiretina are located medially. Upper retinal fibers are located superiorly and lower retinal fibers inferiorly in the optic nerve; this relationship is retained except in the optic tract and lateral geniculate body (LGB).

FIGURE 13.4 The grouping of visual fibers from the retinal quadrants and macular area in the optic nerve, optic tract, lateral geniculate body (LGB), and occipital cortex.
The optic nerve extends from the retina to the optic chiasm; it is approximately 5 cm long. It is conventionally divided into four portions: intraocular (1 mm; the disc), intraorbital (about 25 mm), intracanalicular (about 9 mm), and intracranial (12 to 16 mm). The nerve is organized into 400 to 600 fascicles separated by connective tissue septa. The intraorbital portion is surrounded by fat (Figure 13.5).

FIGURE 13.5 Optic nerve exposed from above with fat and the roof and lateral wall removed. The intraocular segment (a) is within the globe. The intraorbital segment (b) runs through the orbit to the entrance of the optic canal depicted by the left-most blue dot. The short intracanalicular segment (c) courses between the two blue dots. The intracranial segment (d) continues to its junction with the optic chiasm (blue bar). (Courtesy Dr. John B. Selhorst)
The intracranial dura is continuous with the investments of the optic nerve; at the posterior globe the dura fuses with Tenon’s capsule, and at the optic foramen it is adherent to the periosteum. The pia and arachnoid also continue from the brain and envelop the optic nerve. They fuse with the sclera where the nerve terminates. The intracranial meninges extend forward along the optic nerves for a variable distance, forming the vaginal sheaths (Figure 13.2). Through these sheaths, the intracranial subarachnoid space continues along the nerves and may transmit increased intracranial pressure, causing papilledema. Variations in vaginal sheath anatomy may explain the occasional asymmetry of papilledema. Decompression of the optic nerves by opening the sheaths is sometimes done to treat papilledema that threatens vision. The intervaginal space lies between the dura and the pia, divided by the arachnoid into a small subdural and a larger sub-arachnoid space. The ophthalmic artery, the ciliary ganglion and nerves, and the nerves to the extraocular muscles lie close to the optic nerve in the orbital apex. The intraorbital optic nerve is sinuous, with about 8 mm of redundant length to accommodate eye movement. This excess of length allows about 9 mm of proptosis before the nerve begins to tether.
In the peripheral portion of the nerve, near the eye, the PMB is positioned laterally and slightly inferiorly; this separates the temporal fibers into dorsal and ventral quadrants. These in turn crowd and somewhat displace the nasal quadrants (Figure 13.4). As the nerve approaches the chiasm, the PMB moves toward its center.
The intracanalicular portion of the optic nerve begins as it traverses the optic foramen at the orbital apex. The orbital opening of the canal is a vertical ellipse; the intracranial end is a horizontal ellipse. The intracanalicular portion is fixed tightly inside the optic canal with little room to move; intracanalicular lesions can compress the optic nerve while they are still small and difficult to visualize on imaging studies (the “impossible meningioma”). The ophthalmic artery and some filaments of the sympathetic carotid plexus accompany the nerve through the canal.
After traversing the orbit and optic canal, the two optic nerves exit from the optic canals and rise at an angle of about 45 degrees to unite at the optic chiasm, so named because of its resemblance to the Greek letter chi (c) (Figure 13.6). The orbital surface of the frontal lobes lies just above the intracranial optic nerves. The chiasm typically lies about 10 mm above the pituitary gland, separated by the suprasellar cistern. Fibers from the temporal retina continue directly back to enter the ipsilateral optic tract. Fibers from the nasal retina decussate to enter the opposite optic tract.

FIGURE 13.6 The macular fibers decussate as a separate compact bundle, inferior retinal (superior visual field [VF]) fibers cross inferiorly, and superior retinal (inferior VF) fibers superiorly. Masses impinging from below (e.g., pituitary adenoma) tend to cause early defects in the superior temporal fields; masses impinging from above (e.g., craniopharyngioma) tend to cause early defects in the inferior temporal fields.
In 80% of the population, the chiasm rests directly above the sella. In 10%, the chiasm sits forward over the tuberculum sellae with short optic nerves and long optic tracts (prefixed); in the other 10%, the chiasm sits posteriorly over the dorsum sellae with long optic nerves and short optic tracts (postfixed) (Figure 13.7). The position of the chiasm in relation to the sella and the neoplasia-prone pituitary gland influences the clinical presentation of masses in the region.

FIGURE 13.7 The normal position of the chiasm is shown in the top drawing. When the chiasm is prefixed, the optic nerves are short, the chiasm sits forward over the sella, and the optic tracts are long. When the chiasm is postfixed, the optic nerves are long, the chiasm sits posteriorly over the sella, and the optic tracts are short.
The basic scheme of the chiasm with temporal hemiretinal fibers continuing ipsilaterally and nasal hemiretinal fibers decussating is straightforward (Figure 13.8). But there are intricacies in the chiasmal crossing. In the process of decussating, fibers from the inferior nasal quadrant loop forward into the opposite optic nerve for a short distance before turning back again, forming Wilbrand’s knee (see junctional scotoma in “Scotomas” section) (Figure 13.9). In addition, some of the upper nasal fibers loop back briefly into the ipsilateral optic tract before decussation. In the chiasm, the fibers from the upper retinal quadrants lie superior and those from the lower quadrants inferior (Figure 13.5). Inferior nasal fibers decussate anteriorly and inferiorly in the chiasm, while superior nasal fibers cross posteriorly and superiorly, accounting for the difference in the pattern of evolution of the field defect in infrachiasmatic versus suprachiasmatic lesions (Figure 13.5). Macular fibers more or less decussate as a group, forming a miniature chiasm within the chiasm, primarily in the posterior superior portion.

FIGURE 13.8 The course of the visual fibers from the retina to the occipital cortex. A–G show the sites of various lesions that may affect the fields of vision.

FIGURE 13.9 A mass impinging on the optic nerve at its junction with the chiasm, producing a junctional scotoma.
The cavernous sinuses and carotid siphons lie just lateral to the chiasm on either side. The anterior cerebral and anterior communicating arteries are in front and above, and the third ventricle and hypothalamus are behind and above. The sella turcica and sphenoid sinus lie below. The circle of Willis lies above, sending numerous small perforators to supply the chiasm. The ophthalmic artery runs alongside the optic nerve within the same dural sheath through the canal and orbit. About 8 to 12 mm posterior to the globe, the artery enters the nerve and runs along its center to the optic disc, where it becomes the central retinal artery, which pierces the nerve and runs forward onto the disc. The central retinal artery divides at the disc head into superior and inferior branches, which supply the retina. Other terminal branches of the ophthalmic, the short posterior ciliary arteries and choroidal vessels, form an arterial network, the circle of Zinn-Haller, which supplies the disc; the central retinal artery makes only a minimal contribution to the vascular supply of the optic disc.
Posterior to the optic chiasm, the uncrossed fibers from the ipsilateral temporal hemiretina and the crossed fibers from the contralateral nasal hemiretina form the optic tract. About 55% of the axons of the optic tract arise from the contralateral nasal retina and 45% from the ipsilateral temporal retina, which roughly corresponds to the ratio of the area of the temporal field to the nasal field. The tracts contain approximately 80% visual afferents and 20% pupillary afferents. The tracts extend from the chiasm to the LGB, where the majority of fibers terminate. Retinotopic organization is maintained in the optic tract, but the orientation changes. There is a gradual inward rotation, so fibers from the upper retina assume a medial position, while those from the inferior retina lie lateral. Fibers of the PMB gradually assume a dorsal and lateral position, wedged between the upper and lower retinal fibers (Figure 13.4). The retinotopic organization in optic tracts is not as precise as elsewhere, which may contribute to the incongruity of VF defects that are characteristic of optic tract lesions.
Afferents fibers from the pupil leave the tract just anterior to the geniculate to enter the pretectal area of the midbrain (Figure 13.10). The visual afferents synapse in the geniculate on second order neurons, which give rise to the geniculocalcarine pathway (optic radiations).

FIGURE 13.10 Pupillary afferent fibers from the right eye are crossed and uncrossed and run in both optic tracts. They leave the tract before the LGB and send projections to the pretectal region bilaterally. The Edinger-Westphal nucleus sends pupillomotor fibers through the third cranial nerve to the ciliary ganglion, and postganglionic fibers innervate the pupil sphincter. Because of the bilaterality of the pathways, a light stimulus in the right eye causes pupillary constriction in both eyes.
There are six neuronal layers in the LGB, separated by myelinated nerve fibers. Uncrossed fibers from the ipsilateral temporal hemiretina synapse in layers 2, 3, and 5; those from the contralateral nasal hemiretina synapse in layers 1, 4, and 6. Upper retinal fibers remain medial and lower ones lateral (Figure 13.4). Macular fibers occupy an intermediate position in the dorsal, middle, and somewhat caudal portion. The LGB has large magnocellular and small parvicellular neurons. Some of the visual fibers pass over or through the LGB to terminate in the pulvinar of the thalamus, but the significance of this connection has yet to be determined for vision or visual reflexes.
The axons of LGB neurons pass posteriorly to form the geniculocalcarine tract, or optic radiations, and terminate in the calcarine cortex of the occipital lobe (Figure 13.11). Leaving the LGB, the optic radiations pass through the retrolenticular portion of the internal capsule and then fan out. Retinotopically, upper retinal fibers resume an upper, and lower retinal fibers a lower, position in the radiations, with fibers subserving central vision intermediate between the two other bundles. Inferior retinal fibers arch anteriorly into the temporal lobe, sweeping forward and laterally above the inferior horn of the ventricle to run within 5 to 7 cm of the temporal tip, then laterally, down, and backward around the inferior horn. This creates a great arching shape referred to as Meyer’s loop (loop of Meyer and Archambault). The inferior retinal fibers then course through the temporal and occipital lobes. Peripheral retinal fibers loop further forward than macular fibers. Fibers from the superior retina run directly back in the deep parietal lobe in the external sagittal stratum, lateral to the posterior horn of the lateral ventricle. Approaching the occipital lobe, fibers from the upper and lower retina again converge. The primary visual cortex (calcarine area or striate cortex), lies in Brodmann’s area 17 on the medial surface of the occipital lobe. Lower retinal fibers terminate on the lower lip of the calcarine fissure (lingual gyrus) and upper retinal fibers on the upper lip of the calcarine fissure (cuneus). Macular fibers are first lateral and then form the intermediate portion of the geniculocalcarine pathway, continuing to the posterior pole of the occipital lobe. The divergence and convergence of fibers throughout the visual pathway influences the shape and congruity of VF defects, which have localizing value.

FIGURE 13.11 The course of the geniculocalcarine fibers. A. Medial view. B. Inferior view.
Fibers that carry visual impulses from the peripheral portions of the retina terminate on the anterior third or half of the visual cortex of the occipital lobe in concentric zones; macular fibers terminate in the posterior portion (Figure 13.12). The most peripheral parts of the retina are represented most anteriorly in the calcarine cortex; the closer a retinal point lies to the macula, the more posterior its calcarine representation. This culminates in the representation of the macula at the occipital pole. The nasal hemiretina representation extends farther forward than the temporal (the temporal field is more extensive than the nasal), creating a portion of retina for which no homology exits in the opposite eye. This unpaired nasal retina is represented in the most anterior portion of the calcarine cortex, near the area of the tentorium, just outside the binocular VF, which creates an isolated temporal crescent in each VF. Sparing or selective involvement of this monocular temporal crescent has localizing value. The macula has a wider cortical distribution in the striate cortex than in the peripheral retina. It is represented in a wedge-shaped area with its apex anterior. The central 10 to 15 degrees of the VF occupy 50% to 60% of the visual cortex.

FIGURE 13.12 Fibers from the macula synapse in the geniculate and then project to the occipital tip. The most peripherally located retinal ganglion cells synapse in the geniculate and then loop far forward in Meyer’s loop before terminating in the most anterior portion of the calcarine cortex. The most anterior and medial portions of the cortex receive projections from the monocular temporal crescent, which represents the nasal portion of the retina that extends far forward and is the most peripheral part of the retina.
To summarize the retinotopic organization of the visual system: upper retinal fibers remain upper and lower fibers lower throughout except in the tract and LGB where upper becomes medial and lower becomes lateral. The corresponding VF abnormalities can be deduced.
The striate cortex is the sensory visual cortex. It receives afferents via the myelinated stripe of Gennari, which gives this area its distinctive appearance and name. Its physiology is complex. Neurons are arranged in parallel, vertically oriented, ocular dominance columns and complex units called hypercolumns. One hypercolumn can process information from a focal region of the VF. There may be interhemispheric connections through the corpus callosum to synchronize information generated from the two sides. Surrounding the striate cortex are the visual association areas. Area 18, the parastriate or parareceptive cortex, receives and interprets impulses from area 17. Area 19, the peristriate or perireceptive cortex, has connections with areas 17 and 18 and with other portions of the cortex. It functions in more complex visual recognition, perception, revisualization, visual association, size and shape discrimination, color vision, and spatial orientation.
The anterior choroidal artery from the internal carotid and thalamoperforators from the posterior cerebral supply the optic tract. The geniculate is perfused by the anterior choroidal and thalamogeniculate branches from the posterior cerebral. Perhaps because of this redundant blood supply, vascular disease only rarely affects the optic tract or lateral geniculate. Meyer’s loop receives blood supply primarily from the inferior division of the middle cerebral artery, while the optic radiations in the parietal lobe are perfused via the superior division. The occipital lobe is supplied primarily by the posterior cerebral artery. Collaterals from the anterior and middle cerebral may provide additional perfusion to the macular areas at the occipital tip. The parietal smooth pursuit optomotor center and its projections are supplied by the middle cerebral.
Optic Reflexes
Fibers subserving the pupillary light reflex and other optic reflexes pass through the pregeniculate pathways in the same fashion as fibers subserving vision. They leave the optic tract just before it reaches the LGB. Pupillary light reflex fibers travel to the pretectal nuclei, just rostral to the superior colliculus; from the pretectum, axons are sent to synapse on the Edinger-Westphal nuclei. Some light reflex fibers project to the ipsilateral pretectal nucleus to mediate the direct light reflex; others decussate through the posterior commissure to mediate the consensual light reflex (Figure 13.9). Parasympathetic fibers from the Edinger-Westphal nuclei are carried by the oculomotor nerve to the pupillary sphincter.
Fibers controlling somatic visual reflexes, such as turning of the head and eyes toward a visual stimulus synapse in the superior colliculus. From there tectospinal tract fibers descend to more caudal brainstem nuclei to execute the reflex response. The internal corticotectal tract is made up of fibers that run from areas 18 and 19 of the occipital cortex to the superior colliculus to subserve reflex reactions through connections with the eye muscle nuclei and other structures. Fibers that carry impulses having to do with visual-palpebral reflexes (such as blinking in response to light) go to the facial nuclei.
CLINICAL EXAMINATION AND DISORDERS OF FUNCTION
Optic nerve function is tested by examining the various modalities of vision: the visual acuity, the VFs, and special components of vision, such as color vision and day and night vision. The optic nerve is the one cranial nerve that can be visualized directly, and no neurologic, or indeed general, physical examination is complete without an ophthalmoscopic inspection of the optic disc and the retina.
Ideally, the eyes are examined individually. When testing acuity and color vision it is important to occlude the eye not being tested. Before performing the optic nerve examination, look for local ocular abnormalities such as cataract, conjunctival irritation, corneal scarring or opacity, iritis, foreign bodies, photophobia, arcus senilis, glaucoma, or an ocular prosthesis. The presence of a unilateral arcus corneae with ipsilateral carotid disease has been reported. In Wilson’s disease (hepatolenticular degeneration) a yellowish-orange brown coloration 1 to 3 mm wide (Kayser-Fleischer ring) may be seen around the rim of the cornea, more easily in light-eyed individuals. It is due to copper deposition in the posterior stroma and in Descemet’s membrane. It is best seen with a slit lamp. Cataracts may be present in patients with myotonic dystrophy, certain rare hereditary conditions with disturbed lipid or amino acid metabolism, and in many other conditions.
Visual Acuity
Visual acuity is a measure of the eye’s ability to resolve details; it depends on several functions. The intensity threshold reflects the sensitivity of the retina to light; the minimum visibility is the smallest area that can be perceived, and the minimum separability is the ability to recognize the separateness of two close points or lines. Visual acuity charts, the Snellen chart for distance and the near card for near, consist of letters, numbers, or figures that get progressively smaller, and can be read at distances from 10 to 200 ft by normal individuals (Figure 13.13).

FIGURE 13.13 Snellen test chart.
The difference between near and distance vision and between vision with and without correction are points of primarily ophthalmologic interest. For neurologic purposes, only the patient’s bestcorrected visual acuity is pertinent. Refractive errors, media opacities, and similar optometric problems are irrelevant. Acuity is always measured using the patient’s accustomed correction. Ophthalmologists and neuro-ophthalmologists often employ more detailed methods (e.g., full refraction) to clarify the refractive component of a patient’s visual impairment. In infants and children, acuity can be estimated by blink to threat or bright light, following movements, and the pupillary reactions. At the age of 4 months, acuity may be 20/400; it gradually increases, reaching normal levels at about age 5.
For distance vision measurement in the United States, a Snellen chart is placed 20 ft from the patient; at that distance there is relaxation of accommodation, and the light rays are nearly parallel. The eyes are tested separately. In countries using the metric system, the distance is usually given as 6 m. The ability to resolve test characters (optotypes) approximately 1-in high at 20 ft is normal (20/20 or 6/6) visual acuity. These characters subtend 5 minutes of visual arc at the eye; the components of the characters (e.g., the crossbar on the A) subtend 1 minute of arc. The acuity is the line where more than half of the characters are accurately read. If the patient can read the 20/30 line and two characters on the 20/25 line, the notation is 20/30 + 2. By conventional notation, the distance from the test chart, 20 or 6, is the numerator, and the distance at which the smallest type read by the patient should be seen by a person with normal acuity is the denominator. An acuity of 20/40 (6/12) means the individual must move in to 20 ft to read letters a normal person can read at 40 ft. This does not mean the patient’s acuity is one half of normal. In fact, an individual with a distance acuity of 20/40 has only a 16.4% loss of vision.
Since few neurology clinics, offices, or hospital rooms have 20-ft eye lanes, testing is commonly done at a closer distance. Neurologists frequently assess vision with a near card. Though examination of distance vision is preferable, the requisite devices are generally not at hand. There are pocket cards designed for testing at 6 ft, a convenient distance that usually eliminates the need for presbyopic correction. Near vision is tested with a near card, such as the Rosenbaum pocket vision screening card, held at the near point (14 in or 35.5 cm). Jaeger reading cards are still used occasionally (Box 13.1). Good lighting is essential. A penlight shone directly on the line being read is useful for bedside testing.
Jaeger Notation
Jaeger’s test types are ordinary printer’s types, graded from fine (Jaeger 0) to coarse, also used for near testing. The physical optics of the Jaeger system are crude. The numbers refer to the boxes in the Austrian print shop from which Jaeger selected the type in 1854. Jaeger 0 corresponds approximately to an acuity of 20/20. As a rough approximation of near vision, the examiner may use different sizes of ordinary print. Newspaper want-ad text is approximately J-0, regular newsprint J-6, and newspaper headlines J-17.
If the patient cannot read the 20/200 line at 20 ft, the distance may be shortened and the fraction adjusted. Ability to read the line at 5 ft is vision of 5/200, equivalent to 20/800. Vision worse than the measurable 20/800 is described as counts fingers (CFs), hand motion (HM), light perception (LP), or no light perception (NLP). The average finger is approximately the same size as the 20/200 character, so ability to count fingers at 5 ft is equivalent to an acuity of 20/800.
When a patient has impaired vision, an attempt should be made to exclude refractive error by any available means. If the patient has corrective lenses, they should be worn. In the absence of correction, improvement of vision by looking through a pinhole suggests impairment related to a refractive error. Commercial multi-pinhole devices are available. A substitute can be made by making three or four holes with a pin in a 3 × 5 card in a circle about the size of a quarter. The multiple pinholes help the patient locate one. The patient should then attempt to read further down the acuity card through the pinhole. The pinhole permits only central light rays to enter the eye. These are less likely to be disrupted by refractive errors such as presbyopia and astigmatism. If a pinhole was used, make some notation, such as 20/20 (ph). If the visual impairment is due to a neurologic process, such as optic neuritis (ON), vision will not improve with a pinhole. Under some circumstances, such as with opacities in the media (e.g., cataract), vision may get worse with pinhole.
Suspected functional visual loss due to hysteria or malingering is best evaluated by an ophthalmologist, who has the proper tools to answer the question. Clever and determined patients with functional visual loss present a major challenge. There may be certain clues (Box 13.2).
Nonorganic Visual Loss
A truly blind person can sign his name without difficulty. A functionally blind patient often cannot. A truly blind person asked to look at his hand will look wherever proprioception tells him his hand should be; a functionally blind person may gaze in any direction and perhaps never where the hand actually is (Schmidt-Rimpler test). A truly blind person can touch his forefingers together without difficulty; a functionally blind person may make half-hearted inaccurate thrusts. The presence of normal visual, menace, fixation, and emergency light reflexes (see Chapter 16) excludes organic blindness. A functionally blind person ignorant of the laws of reflection may have much improved vision reading the image of an acuity chart held to his chest in a mirror 10 ft away compared to reading the actual chart at 20 ft; the acuity in fact should be the same. Some patients with functional blindness can suppress optokinetic nystagmus (OKN) responses and the visual evoked response (VER). An excellent test is to have the patient look into a large mirror that can be held and moved. Tilting and moving the mirror will elicit OKN responses because the entire visual environment is moving. The patient cannot suppress or “blur out” by willfully failing to fixate on a single target, as he may be able to do with OKN or VER.
The term amblyopia refers to impaired vision due to an organic process in the absence of a demonstrable lesion. The mechanism is poorly understood. Suppression amblyopia is the visual impairment in one eye due to preferential use of the opposite eye in a patient with congenital strabismus. Suppression amblyopia is also referred to as amblyopia ex anopia (amblyopia from disuse). Many other varieties of amblyopia have been described, including alcoholic, toxic, traumatic, and uremic amblyopia. Amaurosis means blindness of any type, but in general usage it means blindness without primary eye disease, or loss of vision secondary to disease of the optic nerve or brain.
Color Vision; Day and Night Vision
Color blindness (achromatopsia) is an X-linked condition present in about 3% to 4% of males. Disturbances of color vision may also occur in neurologic conditions. Loss of color vision may precede other visual deficits. Color deficits may be partial or total. Color plates or pseudoisochromatic plates (Ishihara, Hardy-Ritter-Rand, or similar) formally and quantitatively assess color vision. Having the patient identify the colors in a fabric, such as a tie or a dress, can provide a crude estimate.
Fading or bleaching of colors is a real but uncommon complaint in optic nerve disease. Red perception is usually lost first. Desaturation to red, or red washout, describes a graying down or loss of intensity of red. The bright red cap on a bottle of mydriatic drops is a common test object. The patient compares the brightness or redness in right versus left hemifields, temporal versus nasal hemifields, or central versus peripheral fields. No right/left or temporal/nasal desaturation to red occurs normally. Red does normally look brighter in the center of the VF than off center; reversal of this pattern suggests impairment of central vision. The normal red changes to orange to yellow to colorless as color perception is lost. Because optic neuropathies affect macular fibers, patients lose the ability to read pseudoisochromatic plates. The flight of colors phenomenon is the series of color perceptions that follows shining a bright light into the eye. With impaired color vision, the flight of colors may be reduced or absent. Patients may also compare the brightness or intensity of an examining light in one eye versus the other. A diminution of brightness on one side suggests optic nerve dysfunction; it is sometimes referred to as a subjective afferent pupillary defect (APD), relative APD, or Marcus-Gunn pupil. Its significance is the same as for red desaturation. The APD is discussed in more detail in Chapter 14.
Day blindness (hemeralopia) is a condition in which vision is better in dim lighting than in bright. It occurs in various conditions causing a central scotoma, in early cataracts; it is a rare side effect of trimethadione. Night blindness (nyctalopia) is much poorer vision in feeble illumination than occurs normally. It is common in retinitis pigmentosa and can occur in chronic alcoholism, Leber’s hereditary optic neuropathy (LHON), and xerophthalmia due to vitamin A deficiency.
The Visual Fields
The VF examination is a very important and, unfortunately, often omitted part of the neurologic examination. The VF is the limit of peripheral vision, the area in which an object can be seen while the eye remains fixed. Macular vision is sharp. Peripheral images are not as distinct, and objects are more visible if they are moving. The normal VF extends to 90 to 100 degrees temporally, about 60 degrees nasally, 50 to 60 degrees superiorly, and 60 to 75 degrees inferiorly. The field is wider in the inferior and temporal quadrants than in the superior and nasal quadrants (Figure 13.14). There are individual variations in the field of vision, dependent to some extent on the facial configuration, the shape of the orbit, the position of the eye in the orbit, the width of the palpebral fissure, and the amount of brow projection or the size of the nose. However, these changes are seldom clinically relevant. With binocular vision, the VFs of the two eyes overlap except for the unpaired temporal crescent extending from 60 to 90 degrees on the horizontal meridian, which is seen by one eye only. The monocular temporal crescent exists because of the anatomy of the retina. The nasal retina extends farther forward, more peripherally, than the temporal. This is the true reason that the temporal VF is more expansive, not because the nose is blocking the nasal field.

FIGURE 13.14 The normal VFs.
VF examination results are most accurate in an individual who is alert and cooperative and will maintain fixation. Wandering of the eye impairs the evaluation. Crude assessment is possible even in uncooperative patients if the target is interesting enough (e.g., food or paper money). Fatigue and weakness may lengthen the latency between perception of the test object and the response to it, giving a false impression of VF deficit. Close cooperation, good fixation, and adequate illumination are essential for mapping of the blind spot and delineation of scotomas.
Clinicians use several different methods for VF evaluation. The time and energy expended on bedside confrontation testing depends on the patient’s history and on the facilities available for formal field testing with tangent (Bjerrum) screen (central 30 degrees) or perimetry (entire field). Even sophisticated confrontation testing cannot approach the accuracy of formal fields.
The confrontation VF exam can be tailored to the circumstances and done as superficially or as thoroughly as the situation requires. For a demonstration of confrontation VF examination technique see http://video.google.com/videoplay?docid=6208859529011473674 and http://video.google.com/videoplay?docid=8745940776849382119. Sophisticated bedside techniques can explore the VFs in detail if circumstances warrant. If the patient has no specific visual complaint, and if other aspects of the history and examination do not suggest a field defect is likely, then a screening exam is appropriate. This can be accomplished rapidly and with great sensitivity using small amplitude finger movements in the far periphery of the VF. Recall that the VFs extend temporally to 90+ degrees. Extending elbows and index fingers, the examiner should position the fingers nearly directly lateral to the lateral canthus at a distance of about 24 in. Superficially, this appears to be a binocular examination, but, properly placed, the finger targets are actually in the unpaired monocular temporal crescent part of the VF. With the targets positioned, make a small amplitude flexion movement with the tip of one index finger, perhaps 2 cm in amplitude. Have the patient “point to the finger that moves.” This language is more efficient than attempting a right-left verbal description where the patient’s and examiner’s rights and lefts are reversed. Stimuli should be delivered in each upper quadrant individually, then both together, and then similarly for the lower quadrants. Including bilateral simultaneous stimuli is necessary to detect subtle defects, which may be manifested only by extinction of one stimulus on double simultaneous stimulation. This technique of small finger movements in the far periphery in both upper and lower quadrants is an excellent screen; when properly done, even binocularly, this technique misses few VF defects. Always bear in mind that primary ophthalmologic disorders such as glaucoma, diabetic retinopathy, and retinal detachment can also alter the VFs.
With any hint of abnormality, or if the patient has or could be expected to have a visual problem, higher-level testing is in order. Examining monocularly, techniques include having the patient assess the brightness and clarity of the examiner’s hands as they are held in the right and left hemifields, in both upper and lower quadrants, or having the patient count fingers fleetingly presented in various parts of the field.
More exacting techniques compare the patient’s field dimensions with the examiner’s, using various targets—still or moving fingers, the head of a cotton swab, colored pinheads, or similar objects. Impairment of color perception also occurs with lesions of the posterior visual pathways. Loss of VF to testing with a red object may be apparent even when the fields are intact to a white object. Positioning the patient and examiner at the same eye level, and gazing eyeball to eyeball over an 18- to 24-in span, targets introduced midway between and brought into the VF along various meridians should appear to both people simultaneously in all parts of the field except temporally, where the examiner must simply develop a feel for the extent of a normal field (Figure 13.15).

FIGURE 13.15 Confrontation method of testing the VFs.








