Fig. 7.1.
Considered an anxiety spectrum disorder, obsessive-compulsive disorder (OCD) shows clinical heterogeneity and substantial overlap with other psychiatric disorders
The Obsessive-compulsive spectrum disorders (OCSD) is a grouping of human psychiatric disorders that involve compulsive and/or impulsive symptoms (6, 7). The OCSD include trichotillomania, hypochondriasis, self-harm disorders, tic disorders, body dysmorphic disorder, and eating disorders, in addition to OCD (7) (Fig. 7.1). While it is has been argued that these disorders may in fact be related to OCD, the basis for this classification is purely behavioral (i.e., the involvement of impulsive/compulsive symptoms) without necessarily having a basis in shared etiology, pathophysiology, or treatment profile. As these biological features largely remain unknown, the exact membership in the OCSD family and the classification of the entire group into anxiety disorders remain open to debate (1, 8, 9). Indeed, as Fineberg et al. (1) note, “similarities in phenomenology and comorbidity rates could argue equally well for inclusion of OCD into affective disorders, psychotic disorders, and, even, addiction.”
The discussion on how to classify OCD and other disorders that are potentially related has produced numerous different hypotheses regarding the proper segmentation and optimal diagnostic rubric. Many subtypes of the disorder have been proposed, even for disorders not traditionally paired to OCD, such as autism and Asperger’s Syndrome (10). Some authors posit that typical clinical behaviors are being misdiagnosed due to an overlap in symptomology between the disorders (11), making it less likely for the patient to receive relevant treatment. Similarly, other researchers are investigating the possibility that many of the symptoms that have been intrinsic to OCD, such as hoarding behaviors, could be reinterpreted as distinct syndromes (12). Although there is disagreement on the specific nosology of OCD, it is understood that the examination using animal models will play a crucial role in determining the etiology of the disorder, and lead to a more sophisticated understanding of its effects through investigations of its neurophysiological substrates (see further).
2 Neurobiology of OCD
Overall, clinical studies suggest a heterogeneous mechanism for OCD/OCSD, involving a variety of neurochemical and genetic pathways (5, 9, 13). Importantly, twice as many patients with OCD have a comorbid disorder than OCD alone (1). Disorders commonly associated with OCD include other members of OCSD, depression, and anxiety disorders (particularly, major depressive disorder and social phobias) (1, 3). Due to the high rate of co-occurrence between OCD and OCSD, it has been suggested that they share a common genetic basis (9). While OCD can be heritable in families, as many familial and twin studies have shown, little is known about the genetic mechanisms underlying OCD (5).
The current understanding of OCD pathogenesis has implicated several brain regions. For example, abnormal metabolic activity has been observed in OCD patients in the orbitofrontal cortex, caudate nucleus, and anterior caudal and cingulate medial prefrontal cortex (2). Additionally, OCD patients have elevated activity at rest in the basal ganglia (2).
In general, there are two leading theories regarding the biochemical mechanism of OCD. The serotonin hypothesis arose about 50 years ago when it was noted that serotonin reuptake inhibitors had antiobsessional properties (5). While there is evidence that serotonin dysfunction may play a role in OCD, 40% of patients receiving selective serotonin reuptake inhibitor (SSRI) treatment did not show any clinical improvement (5). Therefore, it has been suggested that only half of the variability in OCD can be accounted for by dysfunction of the serotonergic system (5).
The alternative to the serotonin hypothesis is the dopamine hypothesis, which applies specifically to those forms of OCD related to tic disorders (especially Tourette syndrome), schizotypal personality, or poor insight (5). For example, there is evidence that Tourette syndrome is linked to dopamine dysfunction, and a majority of patients with comorbid OCD and tic disorder respond better to dopamine or dopamine/serotonin treatment than to serotonin treatment alone (5). Because the serotonin and dopamine systems are closely linked, it is possible that both systems are involved in OCD pathogenesis, an idea supported by the cases of de novo OCD that arise in patients being treated for other conditions with antipsychotics with combined dopamine and serotonin reuptake effects (5).
3 Translational Approaches and Experimental Models
The use of animal models in biological psychiatry has become an important direction of research (14, 15). With high construct, predictive, and face validity, animal models allow the testing of etiologic and physiological theories of brain disorders (16). Ethological models became particularly useful for the neurobiological mechanisms of OCD. While some symptoms of OCD cannot be directly observed in animal models (e.g., cognitive obsessions), many repetitive human behaviors are translatable into animal phenotypes (Table 7.1). For example, tail chasing, weaving, fur chewing, excessive grooming, cleaning, pecking, food restriction-induced hyperactivity, reward alternation, excessive lever pressing, barbering, marble burying, acral lick dermatitis, and feather plucking, can be either categorized as naturally occurring repetitive stereotypic behaviors, or instinctive stress-induced motor behaviors (16).
Table 7.1
Parallels between some animal models and human obsessive-compulsive disorder (OCD)
Types of human OCD-like behaviors | Relevant animal behavioral phenotypes | References |
|---|---|---|
Checking | Compulsive checking (Holeboard) | (46) |
Hoarding | Aberrant nest-building, eating behaviors | (19) |
Washing | Aberrant grooming Barbering | |
Ordering | Cognitive inflexibility | (36) |
Other examples of OCD-like behaviors in animals may include excessive or inappropriate variations in water-drinking, attack behaviors, territorial displays, chewing, vocalizations, pacing, freezing, foraging, nest-building, or wheel-running (17). Alterations of these behaviors by pharmacological agents, such as selective serotonin reuptake inhibitors (SSRIs), further supports a strong correlation between animal OCD-like behaviors and the respective human phenotypes. Several experimental models relevant to OCD have been described in the literature, and will be briefly summarized here.
3.1 Marble Burying
The use of nesting material to cover potentially dangerous objects is a commonly observed behavior in mice (18, 19), which was originally utilized in screens for anxiolytic drugs (16, 18, 19). Glass marbles have traditionally been used as a burial-evoking stimulus. Multiple factors have been cited for putting forward marble burying as a model of OCD. These include the observation that mice do not demonstrate avoidance of the marbles (implying the objects do not evoke fear or anxiety), that animals do not grow habituated to their presence (suggesting that the behavior is not due to novelty, the efficacy of SSRIs in reducing burying but not locomotor behavior, and the tendency of the behavior to become excessive (16, 18, 19). It has been suggested that marble burying results from an inability to achieve a sense of task completion. This hypothesis proposes that burying begins as an appropriate investigative behavior, however, as the marbles are nonreactive and provide no stimulus to ending the investigation, the animal becomes frustrated and compulsive burying results (16, 18).
3.2 Barbering
Barbering, wherein a mouse plucks its own hair or whiskers or its cagemates’, is a common behavior in the laboratory animals (20–23), that has been suggested as an animal model for trichotillomania (24–26). Similarities between barbering in mice and human trichotillomania include patterning of hair pulling around the scalp, eyes, and genitals, increased prevalence in breeding animals, female bias, pubertal onset, and genetic contribution (24).
3.3 Nest-Building
Nest-building is an innate behavior in mice that does not require training in order to produce robust results. Nearly all mice given a gauze nestlet to shred, use the material to build round or oval nests (19). Serotonin and norepinephrine uptake inhibitors, as well as GABAergic anxiolytics, have been shown to reduce nest-building at doses that do not affect motor activity (19). The effectiveness of these drugs, which are commonly used in the treatment of OCD patients, suggests that nest-building may be a relevant model of OCD.
3.4 Grooming Behavior
Care of the body surface is an innate behavior in rodents and many other animal species (27–29). In laboratory rodents, grooming has been identified as a complex patterned behavior which can be divided into distinct stages (30–34), and which is highly sensitive to stressors, psychotropic drugs, and genetic manipulations (28, 35). Ethological analysis of grooming assesses an animal’s adherence to the stereotyped grooming pattern as well as other measures including regional distribution and interruptions of grooming bouts (33, 35). Due to its rigid patterning and potential to become excessive, grooming has been suggested as a potential model for OCD (see Fig. 7.2 and (33, 35) for details).
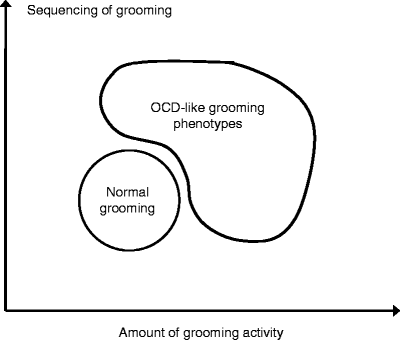

Fig. 7.2.
Potential utility of animal pathological grooming behavior as a model of human obsessive-compulsive disorder (OCD). OCD-like grooming phenotypes, characterized by excessive grooming activity and increased rigidity of grooming sequence, model human OCD behaviors. As grooming activity decreases and sequencing becomes less rigid, this models normal human behavior.
3.5 Spatial Alternation
In spatial alternation tasks, such as the T or Y-maze, some directional preference by test rodents can be expected until the desired behavior has been sufficiently rewarded and reinforced. However, in a small percentage of cases, persistence in preference may remain despite vigorous training, which may be used as a model compulsive behavior seen in the OCD patients (36). Interestingly, Tsaltas et al. (36) have put forward spatial alternation as a low anxiety model for OCD, which is sensitive to serotonergic manipulations commonly used in treating OCD.
4 Genetic Models of OCSD
As already mentioned, genetic factors also play a key role in the OCD-like pathogenesis in humans. Several lines of animal research seem to support this notion. For example, serotonin transporter (SERT) has long been implicated in human anxiety and OCD (37, 38), whereas SERT knockout rodents are extensively used as genetic models of affective disorders (39–44). In several different tests, SERT knockout mice showed greater prevalence of horizontal over the vertical dimension of their exploration, and consistently displayed increased turning and meandering behavior (43, 45). Interestingly, SERT knockout rats showed similar “high-turning” phenotype (Homberg 2008, personal communication), potentially representing a common perseverance-like phenotype (45). In line with this, although stereotypic chewing and grooming behaviors were unaltered in SERT knockout mice (43), SERT knockout rats did display increased grooming behavior (Homberg 2008, personal communication). Collectively, these observations suggest that SERT deficit in rodents may be associated with some alterations in the OCD-related domain.
Similarly, serotonin 5-HT2C receptor knockout mice may also be utilized as a model of OCD in animals, since these mice exhibit increased chewing and head dipping. Specifically, 5-HT2C knockout mice compulsively chew nonnutritive substances, leaving significantly fewer ragged edges than control subjects (46). This abnormal oral behavior is complemented by “mental rigidity,” manifested in a slower habituation of head dipping into a hole in the center of an elevated square board. While clinical evidence implicating the serotonin system in OCD is limited, studies reveal increased fluid levels of a serotonin metabolite 5-HIAA in the cerebral spinal fluid of OCD human patients, thereby supporting the likelihood of a correlation between serotonin and OCD (18, 46).
Recent studies found that a targeted deletion of Sapap3 (a gene encoding protein highly expressed in excitatory synapses of the striatum) induces pronounced OCD-like behaviors in mice (47). Sapap3-mutant mice display excessive and self-injurious behaviors, including self-inflicted facial lesions. Additionally, behavioral tests in these mutants indicate significantly increased duration of grooming and a greater number of grooming bouts, even during periods of the day generally associated with sleep. This compulsive-like behavior was accompanied by an increase in anxiety levels in the open field test and light-dark chamber. The selective expression of Sapap3 rescues these behavioral deficits, supporting the role of excitatory transmission at cortico-striatal synapses in OCD (47).
Furthermore, as the role of dopamine in OCD has been researched extensively, dopamine transporter (DAT) knockdown mice display longer grooming bouts, initiate more syntactic grooming chains, and are more likely to complete syntactic chains once started, compared to the wild-type mice (18). Hyper-dopaminergic mutant mice display significantly strengthened grooming chains which are more resistant to interruption (33). This “sequential super-stereotypy” may translate to frequently observed rigidity in patterning and sequencing in human OCD. In fact, the basal ganglia, commonly implicated in OCD, are also thought to modulate the serial patterning of grooming chains (18).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




