CHAPTER 26 Alcohol-Related Disorders
OVERVIEW
Alcohol is an ambiguous molecule1 often referred to as “man’s oldest friend and oldest enemy.”2 Compared to more structurally complicated substances, such as cannabis or heroin, beverage alcohol (ethanol) possesses a simple chemical structure (C2H5OH) that belies the complexities of its medical, psychological, and social impact.
For most drinkers, its action on the central nervous system (CNS) leads to pleasant subjective experiences that are often encouraged and enhanced by social contexts and by customs. However, if too high a dose is imbibed too rapidly, acute intoxication occurs (leading to a predictable sequence of behavioral disinhibition and cognitive and motor impairments). If a large quantity is consumed, and especially when rapidly consumed,3 alcohol-induced amnesia (“blackouts”) can occur.
In individuals who drink heavily, varying degrees of acute residual lethargy, dehydration, and nausea occur once alcohol is metabolized and eliminated from the body. Carried further, frequent and intense exposure to excessive amounts of alcohol is associated with a wide range of social, legal, and medical problems (including alcohol use disorders; i.e., abuse and dependence). Some common consequences of alcohol misuse include motor vehicle and other accidents, violence and vandalism, unwanted sexual experiences, liver and cardiovascular diseases, cancers, fetal alcohol spectrum disorders, depression, panic attacks, and suicide.4 It is estimated that alcohol causes about 20% to 30% of esophageal cancer, liver cancer, cirrhosis of the liver, homicide, epileptic seizures, and motor vehicle accidents worldwide.5 Excessive or risky alcohol consumption is the third leading cause of death in the United States, accounting for approximately 85,000 deaths annually.6 The economic burden attributed to alcohol-related problems in the United States approaches $200 billion annually.7
DESCRIPTION AND DEFINITION
Alcohol-related disorders are divided into two main groups: alcohol-induced disorders (such as alcohol intoxication, delirium, alcohol withdrawal, persisting alcohol-induced amnestic disorders, and fetal alcohol spectrum disorders) and alcohol use disorders (i.e., abuse and dependence).8
ALCOHOL-INDUCED DISORDERS
Alcohol Intoxication
The action of alcohol on the brain is complex. Low blood alcohol concentrations (BACs) produce activation and disinhibition, whereas higher BACs produce sedation. Behavioral disinhibition is mediated by alcohol’s action as a γ-aminobutyric acid (GABA) agonist, and its interactions with the serotonin system may account for its association with violent behavior. The GABA, N-methyl-d-aspartate (NMDA), and serotonin systems have all been implicated in the escalation to violence.9 Blood alcohol concentrations (BACs) as low as 40 mg/dl may impair memory or lead to an alcoholic blackout, with argumentativeness or assaultiveness developing at levels of 150 to 250 mg/dl and coma or death occurring at 400 to 500 mg/dl (Table 26-1). Yet, chronic alcoholics may be fully alert with a BAC of more than 800 mg/dl, owing to tolerance. Resolution of intoxication follows steady-state kinetics, so that a 70-kg man metabolizes approximately 10 ml of absolute ethanol (or 1.5 to 2 drink equivalents; 1 standard drink = 0.5 oz of whiskey, 4 oz of wine, or 12 oz of beer) per hour.
Table 26-1 Blood Alcohol Concentrations and Their Relationship to Symptoms
| Number of Drinks per Hour* | Approximate Blood Alcohol Concentration (BAC)† | Observable Effects† |
|---|---|---|
| 1-2 | 0.02 | Relaxation, slight body warmth |
| 3 | 0.05 | Sedation, tranquility, slowed reaction time |
| 6 | 0.10 | Slurred speech, poor coordination, slowed thinking |
| 12 | 0.20 | Difficulty walking, double vision, nausea, vomiting |
| 18 | 0.30 | May pass out, tremors, memory loss, cool body temperature |
| 24 | 0.40 | Difficulty breathing, coma, possible death |
| 30 | 0.50 and greater | Death |
* 1 drink = 1.5 fl oz (44 ml) liquor (80 proof) or one glass (5 fl oz [148 ml]) wine or one glass (12 fl oz [355 ml]) beer.
† BAC and the effects of drinking alcohol vary from person to person and depend on body weight, the amount of food eaten while drinking, and each person’s ability to tolerate alcohol.
From http://www.webmd.com/hw/lab_tests/hw3564.asp.
Treatment
If it becomes necessary to sedate an intoxicated individual, one should begin with a smaller than usual dose of benzodiazepines to avoid cumulative effects of alcohol and other sedative-hypnotics. Once the individual’s tolerance has been established, a specific dose can be safely determined. Lorazepam (Ativan) (1 to 2 mg) is effectively absorbed via oral (PO), intramuscular (IM), or intravenous (IV) administration. Diazepam (Valium) and chlordiazepoxide (Librium) are erratically and slowly absorbed after IM administration unless they are given in large, well-perfused sites. When incoordination suggests that the additive effect of a benzodiazepine has produced excessive sedation, it may be advantageous to use haloperidol 5 to 10 mg PO or IM. The initial dose should be followed by a delay of 0.5 to 1 hour before the next dose. If there is no risk of withdrawal, the patient can safely be referred to an outpatient program. Inpatient detoxification is preferable to outpatient care if the patient is psychosocially unstable; has serious medical, neurological, or psychiatric co-morbidity; has previously suffered from complications of withdrawal; or is undergoing his or her first episode of treatment.10 Repeatedly undertreated withdrawal may place the patient at subse-quent risk for withdrawal seizures and for other neurological sequelae through “kindling,” an electrophysiological effect11 that may be mediated, similar to other neurodegenerative effects of ethanol, via the glutamate excitatory neurotransmitter system.12
Alcohol Withdrawal Syndrome
The syndrome of alcohol withdrawal can range from mild discomfort (that requires no medication) to multiorgan failure (that requires intensive care). Uncomplicated withdrawal is surprisingly common and is frequently missed. Although more than 90% of alcoholics in withdrawal need nothing more than supportive treatment, those hospitalized with co-morbid medical conditions have a higher rate of complications.13 The most common features of uncomplicated alcohol withdrawal emerge within hours and resolve after 3 to 5 days. Early features (loss of appetite, irritability, and tremor) of uncomplicated withdrawal symptoms are predictable. A hallmark of the withdrawal syndrome is generalized tremor (fast in frequency and more pronounced when the patient is under stress). This tremor may involve the tongue to such an extent that the patient cannot talk. The lower extremities may tremble so much that the patient cannot walk. The hands and arms may shake so violently that a drinking glass cannot be held without spilling the contents. The patient is hypervigilant, has a pronounced startle response, and complains of insomnia.
Treatment
Rigid adherence to a single protocol for all alcohol withdrawal is unrealistic. Symptom-triggered dosing, in which dosages are individualized and only administered on the appearance of early symptoms, is often recommended. This reduces medication doses by a factor of four, substantially shortens the length of treatment, and shortens symptom duration by a factor of six,10,14 although benefits may be less dramatic in medically ill inpatients.15 Chlordiazepoxide (50 to 100 mg PO) should be given initially, and be followed by 50 to 100 mg every 1 to 2 hours until the patient is sedated and the vital signs are within normal limits. Alternatively, diazepam (10 to 20 mg) may be given initially, and then repeated every 1 to 2 hours until sedation is achieved. Often a first day’s dose of a long-acting benzodiazepine is sufficient for the entire detoxification process because of the self-tapering effect and slow elimination.16 Patients with impaired liver function are better managed with a shorter-acting agent, such as lorazepam 1 to 4 mg PO or IM, or 0.5 mg/min slow IV infusion in severe withdrawal, repeated after 1 to 2 hours, with dose tapering by 25% per day over the subsequent 3 to 6 days.
Alcohol Withdrawal Seizures
Withdrawal seizures occur in roughly 1% of unmedicated alcoholics undergoing withdrawal, although the prevalence is increased in individuals with prior alcohol withdrawal seizures, seizure disorders, and previous brain injuries. Although brain imaging may not be necessary in patients with their first episode,17 seizures during alcohol withdrawal require careful evaluation for other causes. Indications for imaging include neurological and other physical findings suggestive of focal lesions, meningitis, and subarachnoid hemorrhage—all of which may occur in patients with a history of alcohol withdrawal seizures. Multiple prior detoxifications predispose patients to withdrawal seizures more than the quantity or duration of a drinking history, implying a kindling cause.18 Seizures may occur following a rapid drop in the BAC or during the 6 to 24 hours after drinking cessation. Generalized seizures typically occur (i.e., in 75% of cases) in the absence of focal findings, and in individuals with otherwise unremarkable electroencephalogram (EEG) findings. Repeated seizures may occur over a 24-hour period; however, status epilepticus occurs in less than 10% of those who seize.
Treatment
In patients without a prior seizure disorder, diphenylhydantoin offers no benefit over placebo, and given the potential for side effects, diphenylhydantoin is therefore not recommended.18 Also, given that loading with carbamazepine or valproate may not address the rapid time course of withdrawal seizures, the most parsimonious approach remains effective treatment with benzodiazepines. In cases where there is a known seizure disorder, however, conventional management with anticonvulsants is in order.
Alcohol Withdrawal Delirium
Delirium tremens, or “DTs,” the major acute complication of alcohol withdrawal, was renamed “alcohol withdrawal delirium” in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).8 Until open-heart procedures spawned new postoperative deliria, DTs were by far the most frequently encountered delirium in a general hospital, reportedly occurring in 5% of hospitalized alcoholics. Although it was first described in the medical literature more than 150 years ago and has been frequently observed ever since, DTs still go undiagnosed in a large number of cases. It is missed because physicians tend to forget that alcoholism is rampant among people of all backgrounds and appearances. Because deaths have occurred in 10% of patients with untreated alcohol withdrawal delirium and in 25% of those patients with medical or concomitant surgical complications, it is imperative to be on the alert for this life-threatening condition.
It is difficult to predict who will develop DTs. Until a decade ago, DTs rarely developed in patients younger than 30 years of age. This is no longer true. Today the condition is frequently observed in young patients who may have had a decade or more of chronic heavy alcohol consumption. The mechanisms may involve NMDA-glutamate receptor supersensitivity.11 Although delirium is regarded as a withdrawal syndrome, some heavy drinkers fail to develop delirium after sudden withdrawal of ethanol. Infection, head trauma, and poor nutrition are potentially contributing factors to delirium. A history of DTs is an obvious predictor of future DTs.
The incidence of DTs is approximately 5% among hospitalized alcoholics and about 33% in patients with alcohol withdrawal seizures. If DTs do occur, they generally do so between 24 and 72 hours after abstinence begins. There have been reports, however, of cases in which the clinical picture of DTs did not emerge until 7 days after the last drink. The principal features are disorientation (to time, place, or person), tremor, hyperactivity, marked wakefulness, fever, increased autonomic tone, and hallucinations. Hallucinations are generally visual, but they may be tactile (in which case they are probably associated with a peripheral neuritis), olfactory, or auditory. Vestibular disturbances are common and often hallucinatory. The patient may complain of the floor moving or of being on an elevator. The hallucinatory experience is almost always frightening, such as spiders and snakes that may have additional characteristics (e.g., more vivid colors and mice or insects sensed on the skin). Once the condition manifests itself, DTs usually last 2 to 3 days, often resolving suddenly after a night of sound sleep. Should it persist, an infection or subdural hematoma may be the cause. There are, however, a small number of individuals whose course is characterized by relapses with intervals of complete lucidity. These patients offer the clinician the most challenging diagnostic opportunities. As a rule of thumb, it is always wise to include DTs in the list of diagnoses considered whenever delirium appears. Even skilled clinicians are apt to miss the diagnosis of DTs when the patient’s manner, social position, or reputation belies a preconceived and distorted stereotype of an “alcoholic.” The clinician is also frequently misled when the delirium is intermittent and the patient is examined during a lucid stage. Although a course of intermittent episodes is highly atypical for DTs, it can occur.
Treatment
Prevention is the key. Symptom-triggered dosing for alcohol withdrawal has been shown to reduce DTs more than use of standing benzodiazepine orders in medically ill inpatients.15 As in the treatment of any delirium, the prime concern must be round-the-clock monitoring so that the patient cannot harm himself or herself or others. Although not necessarily suicidal, delirious patients take unpremeditated risks. Falling from windows, slipping down stairs, and walking into objects are common examples. Restraint should be used only for short periods. As required by law, when four-point restraint is used, the patient must be closely observed, and relief must be provided every hour. Usually, physical restraint can be avoided with aggressive pharmacotherapy.
The delayed onset of this hyperarousal state may reflect alcohol’s broad effects across multiple neurotransmitter systems, chief among which may be the NMDA-glutamate system.12 Adrenergic hyperarousal alone appears to be an insufficient explanation so that α-adrenergic agonists (e.g., clonidine and lofexidine) alone are not appropriate. Benzodiazepines alone may not suffice. In rare cases, doses of diazepam in excess of 500 mg/day may prove insufficient. Haloperidol 5 to 10 mg PO or IM may be added and repeated after 1 to 2 hours when psychosis or agitation is present. Propofol may be used in those cases of severe DTs unresponsive to other medications.19
Wernicke-Korsakoff Syndrome
Victor and colleagues,20 in their classic monograph The Wernicke-Korsakoff Syndrome, state that “Wernicke’s encephalopathy and Korsakoff’s syndrome in the alcoholic, nutritionally deprived patient may be regarded as two facets of the same disease. Patients evidence specific central nervous system pathology with resultant profound mental changes.” Although perhaps 5% of alcoholics have this disorder, in 80% of these cases, the diagnosis is missed. In all of the cases reported by Victor and colleagues,20 alcoholism was a serious problem and was almost invariably accompanied by malnutrition. Malnutrition, particularly thiamine deficiency, has been shown to be the essential factor.
Wernicke’s Encephalopathy
Wernicke’s encephalopathy appears suddenly and is characterized by ophthalmoplegia and ataxia followed by mental disturbance. The ocular disturbance, which occurs in only 17% of cases, consists of paresis or paralysis of the external recti, nystagmus, and a disturbance in conjugate gaze. A global confusional state consists of disorientation, unresponsiveness, and derangement of perception and memory. Exhaustion, apathy, dehydration, and profound lethargy are also part of the picture. The patient is apt to be somnolent, confused, and slow to reply, and may fall asleep in midsentence. Once treatment with thiamine is started for Wernicke’s encephalopathy, improvement is often evident in the ocular palsies within hours. Recovery from ocular muscle paralysis is complete within days or weeks. According to Victor and colleagues,20 approximately one-third recovered from the state of global confusion within 6 days of treatment, another third within 1 month, and the remainder within 2 months. The state of global confusion is almost always reversible, in marked contrast to the memory impairment of Korsakoff’s psychosis.
Treatment
Administration of the B vitamin, thiamine (IM or IV), should be routine for all suspected cases of alcohol intoxication and dependence.21 The treatment for Wernicke’s encephalopathy and Korsakoff’s psychosis is identical, and both are medical emergencies. Because subclinical cognitive impairments can occur even in apparently well-nourished patients, routine management should include thiamine, folic acid, and multivitamins with minerals, particularly zinc. Prompt use of vitamins, particularly thiamine, prevents advancement of the disease and reverses at least a portion of the lesions where permanent damage has not yet been done. The response to treatment is therefore an important diagnostic aid. In patients who show only ocular and ataxic signs, the prompt administration of thiamine is crucial in preventing the development of an irreversible and incapacitating amnestic disorder. Treatment consists of 100 mg of thiamine and 1 mg of folic acid (given IV) immediately and 100 mg IM of thiamine each day until a normal diet is resumed, followed by oral doses for 30 days. Parenteral feedings and the administration of B-complex vitamins become necessary if the patient cannot eat. If a rapid heart rate, feeble heart sounds, pulmonary edema, or other signs of myocardial weakness appear, the patient may require digitalis. Because these patients have impaired mental function, nursing personnel should be alerted to the patient’s tendency to wander, to be forgetful, and to become obstreperously psychotic. If the last should occur, benzodiazepines can be given.
Korsakoff’s Psychosis
Korsakoff’s psychosis, also referred to as confabulatory psychosis and alcohol-induced persisting amnestic disorder in DSM-IV,8 is characterized by impaired memory in an otherwise alert and responsive individual. This condition is slow to start and may be the end stage of a lengthy alcohol-dependence process. Hallucinations and delusions are rarely encountered. Curiously, confabulation, long regarded as the hallmark of Korsakoff’s psychosis, was exhibited in only a limited number of cases in the large series collected and studied by Victor and colleagues.20 Most of these patients have diminished spontaneous verbal output, have a limited understanding of the extent of their memory loss, and lack insight into the nature of their illness.
Patients with Korsakoff’s psychosis tend to improve with time. Among Victor and colleagues’ patients,20 21% recovered more or less completely, 26% showed no recovery, and the rest recovered partially.8 During the acute stage, however, there is no way of predicting who will improve and who will not. The EEG may be unremarkable or may show diffuse slowing, and magnetic resonance imaging (MRI) may show changes in the periaqueductal area and medial thalamus.11 The specific memory structures affected in Korsakoff’s psychosis are the medial dorsal nucleus of the thalamus and the hippocampal formations.
Fetal Alcohol Spectrum Disorder
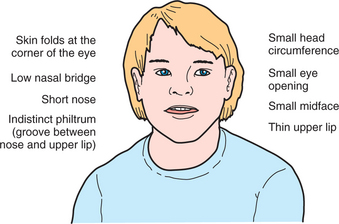
Fetal alcohol spectrum disorder (FASD) is an umbrella term that describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These effects may include physical, mental, behavioral, or learning disabilities with possible life-long implications. Formerly known as “fetal alcohol syndrome,” these disorders can include a set of birth defects caused by heavy consumption of alcohol during pregnancy. Children with this condition typically have facial deformities, a misproportioned head, mental retardation, and behavioral problems (Figure 26-1).
However, even when these abnormalities are not evident, brain damage may still have occurred. Approximately 30% to 40% of all women who drink heavily during pregnancy will have a baby with some degree of FASD. It is the leading cause of preventable mental retardation in the Western Hemisphere. Studies using MRI to view the brains of children with FASD show that brain areas that regulate movement and cognitive processes related to attention, perception, thinking, and memory are particularly sensitive to prenatal alcohol exposure, and that brain size is reduced.4
ALCOHOL USE DISORDERS
Alcohol use disorders are classified into two main categories: “alcohol dependence” and either “alcohol abuse” or “harmful use,” depending on which of the two major diagnostic systems one uses: the International Classification of Diseases, Version 10 (ICD-10; “harmful use”) or the DSM-IV “abuse.”8 Increased collaboration has meant that the DSM and ICD classifications are almost identical in their nomenclature. Importantly, both systems delineate “polythetic” classifications of dependence, since only three from a list of seven symptoms are required to meet a diagnostic threshold. This highlights a degree of heterogeneity within the syndrome that has typological and clinical implications for detecting and treating the disorder. The DSM criteria for dependence and abuse can be seen in Table 26-2.
Table 26-2 The DSM-IV Diagnostic Criteria for Alcohol Use Disorders
The alcohol dependence syndrome was first described in the 1970s22 and has since been validated and generalized to describe the dependence syndrome (also often referred to as “addiction”) across all psychoactive substances. Edwards and Gross22 noted that the dependence syndrome may be recognized by the clustering of certain elements. Not all elements need be present or present to the same degree, but with increasing intensity the syndrome is likely to show logical consistency. It is conceptualized as an integration of physiological and psychological processes that leads to a pattern of heavy alcohol use that is increasingly unresponsive to external circumstances or to adverse consequences. Furthermore, they viewed the syndrome not as an all-or-nothing dichotomy, but as occurring with graded intensity, and its presentation as being influenced by personality, as well as by social and cultural contexts. Their conceptualization also introduced a “biaxial” model with the dependence syndrome constituting one axis and alcohol-related problems the other.
In the United States before DSM-III,23 there was only a single descriptive category, “alcoholism,” which hitherto had been viewed as a personality disorder. DSM-III was influenced by the syndrome and biaxial concepts of Edwards and Gross22 and, consequently, introduced a distinction between “dependence” and “abuse.” DSM-III was the first diagnostic manual of mental disorders in the United States to introduce actual itemized criteria, increasing the reliability of these diagnoses.24
The term alcoholism was originally coined to describe alcohol dependence/addiction and is often still used as an alternative to dependence. However, it is often used more broadly to describe alcohol dependence and alcohol abuse/hazardous use, sometimes without explicit mention of the fact. This variation in usage can be confusing. It may also lead to errors in clinical and scientific communication as it has implications for inferences that are drawn from clinical data and may ultimately affect treatment policy decisions.25 Thus, we believe care should be taken in choosing descriptive terms and in using them accurately and consistently.
As described in Table 26-2, alcohol dependence is characterized by the broad elements of neuroadaptation (tolerance and withdrawal) and an impaired ability to alter or to stop alcohol consumption for very long, despite the personal suffering it causes (impaired control over use). As a construct, assessment of alcohol dependence has been shown to be reliable and to possess good construct and predictive validity. In contrast, the “abuse” category, describing role impairment and other consequences from the misuse of alcohol, has been shown to be a less reliable and valid construct.26 Several studies, for example, have shown that current diagnoses of alcohol dependence using DSM-III-R, DSM-IV, and ICD-10 criteria27 evince highly concordant rates in community samples,28 among demographic subgroups,29 and in cross-national samples.30 Furthermore, the dependence syndrome has good construct and predictive validity and can be reliably measured. The “abuse” category, on the other hand, appears to possess much poorer reliability and construct and predictive validity.26
In the DSM-IV system, these disorders are organized hierarchically, meaning that someone cannot receive a diagnosis of “abuse” if he or she meets criteria for dependence. Interestingly, many who meet criteria for dependence do not meet criteria for “alcohol abuse.” The recent National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) found that 34% of individuals with current alcohol dependence did not meet criteria for alcohol abuse.31 This has important clinical relevance because it indicates that while an individual may not meet criteria for an alcohol “abuse” diagnosis, it does not mean that one should stop the screening/assessment process and assume the individual will not meet criteria for any alcohol use disorder; he or she may still meet criteria for alcohol dependence, and should be assessed for such.
ETIOLOGY, EPIDEMIOLOGY, AND TYPOLOGY
Etiology
The relative contributions of genetic and environmental factors to the manifestation of alcohol use disorders can be expressed as the population-attributable risk percent, meaning the percentage of disease incidence that would be eliminated if the risk factor were removed.32 A genetic heritability estimate for alcohol dependence is sometimes estimated at approximately 50% with the other 50% (equaling “100%”) attributable to “environmental causes.” However, these estimates are misleading since the attributable risks for a complex disease, such as alcohol dependence, can add to well over 100% because the disorder can be avoided in many different ways. These additional percentages can be described as interactions among the various risk factors (e.g., gene-environment interactions). For example, a genetic abnormality may be necessary for a disease to occur, but the disease will not occur without the presence of an environmental risk factor. Thus, the attributable risks for the genetic aberration and the environmental factor would both be 100%. Phenylketonuria is an example of this: the disease can be avoided either by not having the genetic abnormality or by eliminating phenylalanine from the diet.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree