CHAPTER 30 Bipolar Disorder
EPIDEMIOLOGY AND RISK FACTORS
The National Comorbidity Survey—Replication (NCS-R) study, in which a random population-based sample of about 9,000 adults was contacted and screened using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)–based questions, estimated a lifetime prevalence of 1% for bipolar I disorder and 1.1% for bipolar II disorder.1 A previous population-based survey using a validated self-report questionnaire estimated the prevalence of BPD at 3.4% to 3.7%.2 In the NCS-R, the prevalence of “subthreshold” BPD—that is, two or more core features of hypomania, without meeting criteria for BPD—was estimated at 2.4%. With this broader definition, the prevalence of all “bipolar spectrum” disorders reaches 4.4%.
According to the NCS and previous studies, the prevalence for BPD is in general similar between men and women,3 though gender differences may exist in illness features.4 The risk for BPD also appears to be similar across racial groups and geographical regions. For example, epidemiological studies indicate a lifetime prevalence between 0.3% in Taiwan and 1.5% in New Zealand.3 Past studies have also suggested that BPD might be under-recognized among non-Caucasians, because of a tendency instead to diagnose these individuals with schizophrenia. However, the NCS-R survey found no differences in the prevalence of BPD by race/ethnicity or by socioeconomic status (defined by family income).1
A number of putative environmental risks have been described for BPD5; these include pregnancy and obstetrical complications, season of birth (winter or spring birth, perhaps indicating maternal exposure to infection), stressful life events, traumatic brain injuries, and multiple sclerosis (MS). In MS, for example, the prevalence of BPD is roughly doubled6; this increase does not appear to result from adverse effects of pharmacotherapy. The prevalence may also be increased among individuals with certain neurological disorders, including epilepsy. Another intriguing finding is the association between dietary omega-3 fatty acid consumption and risk of mood disorders. Most such studies focus broadly on depressed mood, although one study reporting on data from 18 countries found greater seafood consumption to be associated with a lower risk for BPD.7,8
HISTORICAL CONTEXT
The concept of mood disorders dates back at least to the observations of the ancient Greeks, who recognized melancholia (depression) and mania. Beginning with the Greeks, and continuing through the nineteenth century, multiple authors independently connected the two mood states.9,10 For example, the French physician Jules Baillarger referred to “la folie a double-forme,” the alternation of manic and depressive episodes. Indeed, the concept of a dichotomy between MDD and BPD did not reemerge until the 1960s, and remains a subject of controversy (see the section on bipolar spectrum illness, later in this chapter).
The modern concept of BPD is attributed to Emil Kraepelin, whose text described the principle of opposing mood states, and more broadly distinguished primary affective disorders from primary psychotic disorders, a distinction that still stands in the DSM-IV, despite increasing evidence that this distinction is not absolute (see the section on genetics, later in this chapter). Kraepelin’s description of phenomenology, based on careful longitudinal evaluation, is instantly recognizable to many modern clinicians. However, some of the nuance of Kraepelin’s descriptions has not been transmitted in modern definitions. To cite but one example, the description (by Kraeplin’s student, Weygandt) of multiple categories of mixed states actually presages modern debates about the overlap between rapid mood cycling, mixed manic and depressive presentations, subthreshold mood symptoms, and co-morbid anxiety and psychosis.11
CLINICAL FEATURES AND PHENOMENOLOGY
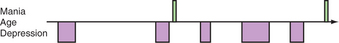
BPD is characterized by the presence of mood episodes—that is, periods of change in mood with associated symptoms. These mood episodes are described as depressive, hypomanic, manic, or mixed depending on the predominant mood and the nature of associated symptoms. Criteria for each mood state are included in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Mood episodes are not in and of themselves diagnoses, but rather they form the building blocks for the diagnosis of mood disorders. The key feature for the diagnosis of BPD is the presence of at least one period of mood elevation or significant irritability meeting criteria for a manic, mixed, or hypomanic episode. These episodes typically recur over time; see Figure 30-1 for a graphical illustration of the course of illness in one patient.
A manic episode is identified when a patient experiences an elevated or irritable mood for at least a week, along with at least three associated symptoms (described in Table 30-1). If the predominant affect is irritable, four rather than three associated symptoms are required. If the symptoms result in hospitalization at any point, the 1-week criterion is not required—for example, a patient hospitalized after 3 days of manic symptoms is still considered to have experienced a manic episode. As with episodes of major depression, DSM-IV-TR criteria also require that symptoms be sufficient to markedly impair occupational or social function, or be associated with psychotic symptoms. Inter-rater reliability of the DSM mania criteria has been shown to be quite high.
Table 30-1 Diagnostic Criteria: Mania
NOTE: Manic-like episodes that are clearly caused by somatic antidepressant treatment (e.g., medication, electroconvulsive therapy, light therapy) should not count toward a diagnosis of Bipolar I Disorder.
From DSM-IV™. American Psychiatric Association, Washington, DC, 1994, p. 332.
Hypomanic symptoms are generally similar to, but less severe and impairing than, manic symptoms. DSM-IV-TR criteria require at least 4 days of mood elevation or irritability, along with associated symptoms. There are three important, but often overlooked, aspects of these criteria that bear highlighting. First, symptoms must be observable by others—that is, a purely subjective report of hypomania is not sufficient for a diagnosis. Second, symptoms represent a change from the individual’s baseline; those who are “always” cheerful, impulsive,and talkative are “not” considered chronically hypomanic (though see the section on hyperthymia, later in this chapter). Third, symptoms by definition do not cause significant functional impairment—hypomanic-like symptoms that lead to loss of a job, for example, could be considered mania. As these criteria may be difficult to operationalize, it is not surprising that inter-rater reliability of hypomania criteria is somewhat lower. Some diagnostic systems propose that 1 to 3 days of hypomanic symptoms are required to identify hypomania12; this approach trades specificity for sensitivity.
A major depressive episode is defined exactly as it is in MDD: the presence of depressed mood or loss of interest, most of the day, more days than not, with additional symptoms that include changes in sleep or appetite, poor self-esteem, feelings of guilt, fatigue, poor concentration, psychomotor agitation or slowing, and thoughts of suicide. (See Chapter 29 for details.) When criteria are met for both a major depressive and a manic episode nearly every day for at least 1 week, an episode is characterized as a mixed state.
Having identified the presence and type of current and past mood episodes, the clinician may then categorize the type of mood disorder and make a diagnosis. These diagnostic features are summarized in Table 30-2. Individuals with at least one manic episode are considered to have bipolar I disorder. Those with at least one hypomanic and one depressive episode, but never a manic episode, are considered to have bipolar II disorder. Individuals who have never experienced a period of hypomania, mania, or a mixed state do not, by DSM-IV criteria, have BPD (see the section on bipolar spectrum illness, later in this chapter). Note that individuals with episodes of hypomania but never manic/mixed states must also have had at least one depressive episode to meet criteria for BPD. In practice, the prevalence of hypomanias without a single depressive episode is quite rare.
Table 30-2 Diagnostic Features of Mood Disorders
| Mania/Mixed | Depression | |
|---|---|---|
| Bipolar I | Yes | Typical but not required |
| Bipolar II | Hypomania only | Yes |
| MDD | Never | Yes |
| Cyclothymia | Never (but periods of elevation) | Symptoms but not full episode within first 2 years |
MDD, Major depressive disorder.
ASSOCIATED ILLNESS FEATURES
Bipolar I versus II
The distinction between bipolar I and II disorder formally entered the American diagnostic system in the DSM-IV, though it was initially described in 1976,13 based on apparent stable differences in course of illness. Indeed, modern studies suggest that transition from bipolar II to bipolar I among adult patients is rare.14 Some studies suggest that bipolar II patients may experience more frequent episodes and greater risk for rapid cycling,15 as well as greater burden of depressive symptoms.16 These differences belie the common misconception that bipolar II is less disabling than bipolar I.
Psychosis
Of note, psychosis is not represented in the diagnostic features for BPD. However, psychotic symptoms are common during both manic/mixed and depressive episodes. A Finnish population-based study found a prevalence of 0.24% for psychotic bipolar I disorder.17 The lifetime prevalence of psychotic symptoms in a cohort of bipolar patients was approximately 40%.18 So-called “mood-congruent” psychotic symptoms are often seen—for example, grandiose delusions during mania or delusions of decay and doom during depression. Psychosis typically resolves along with the mood symptoms, though diagnostic criteria acknowledge that psychotic symptoms may linger beyond the end of the mood episode.
Suicide
Suicidal thoughts and attempts are also not required for a diagnosis of BPD, although they are among the criteria for a depressive episode. In one large cohort of bipolar I and II patients, between 25% and 50% reported at least one lifetime suicide attempt.18 In population-based studies, risk of death from suicide among bipolar patients is estimated at between 10 and 25 times that of the general population,19,20 similar to that observed in MDD.20
Cognitive Symptoms
Increasingly, a subset of patients with BPD has been recognized as experiencing cognitive impairment, both during and outside of mood episodes.21 Such impairment has been difficult to characterize because it is likely multifactorial, but clearly contributes to the profound functional impairments experienced by many bipolar patients.21 Many commonly used pharmacotherapies (including lithium and anticonvulsants) can affect cognition. Likewise, residual mood symptoms, both depressive and manic, may affect cognition—for example, difficulty with concentrating or distractibility may occur. Attention-deficit/hyperactivity disorder (ADHD) has also been suggested to be prevalent among patients with BPD. This finding may represent “true” ADHD, or simply an overlap in the diagnostic criteria of these diagnoses.
Regardless of potential confounding effects, rigorous studies incorporating neuropsychological testing of relatively euthymic patients suggest a plethora of cognitive complaints.21 Because these studies often use different cognitive batteries among relatively small numbers of patients, it is difficult to arrive at a single cognitive profile that is typical for BPD. Typical findings on neuropsychological testing include impairment in attention and executive function and, in some studies, deficits in working or verbal memory.
FEATURES OF LONGITUDINAL COURSE
Age at Onset
More recent studies suggest that the age of onset of BPD may be somewhat earlier than previously appreciated. Unfortunately, most such studies rely on retrospective reporting, and frequently fail to distinguish between the onset of mood symptoms and the onset of a syndromal mood episode. In the NCS-R survey, the mean age at onset for bipolar I disorder (defined syndromally) was 18.2 years, and for bipolar II disorder it was 20.3 years.1 In a large cohort study of adults with BPD, nearly one-third of individuals reported the onset of symptoms before age 13, and another one-third became symptomatic between ages 13 and 18 years.18 In this study, earlier onset was associated with a more chronic and recurrent course, greater functional impairment, and greater Axis I co-morbidity.
Mood Episodes
Traditional emphasis on the episodic structure of BPD has been complemented with a recognition that, for many patients, symptoms may be chronic and persist beyond discrete episodes (see Figure 30-1). Indeed, such symptoms likely contribute substantially to the disability associated with BPD, estimated to be one of the 10 greatest medical causes of disability worldwide.22 In general, while hypomania and mania are considered the defining features of BPD, patients spend a far greater amount of time ill—around two-thirds of the time, in one longitudinal study23—with depressive symptoms.
Up to 40% of bipolar I patients experience a mixed state at some point in their disease course.24 Recently, the concept of subthreshold mixed states has received increasing attention: patients who do not meet the stringent criteria for a mixed state (who do not meet full criteria for both a manic and a depressive episode simultaneously) but nonetheless have some degree of both types of symptoms. Depressive symptoms are common during manic or hypomanic episodes, underscoring the importance of inquiring about both poles. Conversely, during depressive episodes, patients may experience some degree of hypomanic symptoms, such as racing thoughts. The prognostic implications, if any, of these subthreshold mixed states has not been well studied.
Rapid Cycling
DSM-IV-TR criteria describe rapid cycling as a course specifier in BPD—that is, an illness feature that may be present at times but not necessarily throughout the course of the illness. Specifically, individuals with at least four syndromal mood episodes within a single year, separated by full recovery or a switch to the opposite pole, are considered to experience rapid cycling. In one cohort of bipolar patients, prevalence of rapid cycling was 20% assessed retrospectively,25 and rapid cycling was associated with greater illness burden and chronicity.
Antidepressant-Induced Mania/Hypomania
A small number of patients experience the onset of mania or hypomania after initiation of antidepressants. While not a formal category in DSM-IV-TR, some authors refer to this phenomenon as “bipolar III.” The true prevalence and time course of this phenomenon are difficult to estimate, particularly for a switch to hypomania, because in clinical practice, as well as in randomized controlled trials (RCTs), such symptoms of elevated mood may not be aggressively investigated. A patient who sees his or her clinician, 2 weeks after beginning an antidepressant, feeling “great” and congratulating the clinician on the clinician’s excellent skills requires careful questioning about manic/hypomanic symptoms, but more often is congratulated in turn on his or her excellent antidepressant response. In one of the largest prospective antidepressant treatment studies to date in MDD,26 there was little or no evidence of antidepressant-induced mood elevation. Likewise, most randomized trials in MDD report switch-rates of less than 1%.
Seasonality and Climate
A relationship between season and course of illness in BPD has been reported, but its precise nature remains unclear. Multiple studies suggest peaks in admissions for mania, generally occurring in late spring/summer.27–29 One report suggested that most bipolar patients follow one of two patterns: depression in fall or winter, with elevation in spring/summer, or spring-summer depression, with fall-winter mania.30 Most recently, a large cohort study found depression peaks in February and July, but only in BPD-II.31 They found depression to be more prevalent among patients with BPD in more northern regions of the United States.
Changes in Episode Frequency
A persistent belief about BPD dating back to Kraepelin’s descriptions has been that the interval between episodes decreases over time, at least across the first several episodes. This apparent observation contributed in part to earlier hypotheses that posited a “kindling” effect in which episodes beget subsequent episodes. However, it may actually be the result, at least in part, of a statistical artifact first described more than 70 years ago and referred to as “Slater’s fallacy.”32 Indeed, more recent analyses suggest that such worsening with time is not the case for many patients.33
NEUROBIOLOGY AND PATHOPHYSIOLOGY
Hypotheses
A number of overlapping hypotheses guide current research into the neurobiology of BPD. One set of observations relates to the mechanism of action of lithium, known to be an effective treatment for BPD (see Chapter 47). While its therapeutic mechanism is not known, it does interact with two key pathways in cell signaling: inositol-triphosphate (InsP3)–dependent signaling, and the Wnt signaling pathway, the latter because it inhibits glycogen synthesis kinase 3-beta (GSK3B). Both pathways have received increased attention for their potential role in BPD. Other hypotheses of current interest include the role of glial cell abnormalities,34 as well as mitochondrial dysfunction.35
Animal Models
Research into the pathophysiology of BPD has been hindered by the lack of a convincing animal model.35 Perhaps the best-studied model is the mouse psychostimulant model, which is purported to mimic mania, at least in terms of inducing psychomotor agitation. Recently, a second potential mouse model of mania was created by disrupting a gene important in circadian rhythms, CLOCK.36 Mania-like features in these mice include marked decrease in sleep and increase in activity, as well as an increase in the “rewarding” effects of cocaine; most intriguingly, these changes are normalized by the administration of lithium.
Postmortem Studies
Another approach to the study of BPD relies on postmortem studies of brains from bipolar patients. Neuropathological studies suggest decreased density or morphology of oligodendrocytes.37 Other studies examine changes in gene expression. Major caveats with this approach include the sensitivity of these studies to postmortem handling of samples and the mode of death (e.g., duration of agonal period), as well as difficulty in discriminating medication effects from the effects of a primary disease. Still, such studies suggest differences between brains of bipolar patients and normal controls. For example, one small study found down-regulation of genes related to myelination and oligodendrocytes, similar to that observed in the brains of patients with schizophrenia.34
Neuroimaging Studies
Structural brain imaging has identified regional differences in multiple brain structures among bipolar patients, predominantly cortical and subcortical areas implicated in limbic circuitry. While studies of the prefrontal cortex as a whole are inconsistent, a number of reports find decreased volume in individual regions among bipolar patients (reviewed by Strakowski and colleagues38). Among subcortical structures, perhaps the most consistent findings are of increased volume in striatum and amygdala. Notably, data from twin and early-onset cohorts suggest that striatal changes may represent “trait” markers of bipolarity, present early in the disease process.
Functional imaging in BPD is complicated by the need to consider variation in mood states, and particularly by practical difficulties in imaging truly manic patients. Region-specific differences by mood state have been noted in regions of prefrontal cortex; for example, one study found decreased perfusion of subgenual prefrontal cortex during depression and increased perfusion during mania.39 Similar increases have been noted in basal ganglia perfusion among manic patients.40 Studying euthymic bipolar patients provides an opportunity to elucidate potential trait markers of bipolarity. In one such study, on a cognitive task, bipolar patients demonstrated greater activation than healthy controls in regions including the ventrolateral prefrontal cortex and amygdala.41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree