CHAPTER 46 Neurotherapeutics
OVERVIEW
Most patients with psychiatric illness are successfully treated with pharmacotherapy or psychotherapy (or both). However, some of these patients do not respond to these interventions. For example, studies have demonstrated that about 30% to 40% of patients with major depressive disorder (MDD) treated with pharmacotherapy achieve full remission,1 and 10% to 15% experience no symptom improvement.2 In addition, the recently completed Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that with each failed medication trial the remission rate decreased.3 Clearly, alternative therapeutic interventions for patients with no response to these treatments are necessary. This chapter will focus on surgical and device-based treatments that are currently being used, or are under investigation, in attempts to address these treatment-resistant patients. The field associated with these new treatments is often referred to as neurotherapeutics or neuromodulation. Examples of neurotherapeutic interventions include ablative limbic system surgeries (e.g., anterior cingulotomy, subcaudate tractotomy, anterior capsulotomy, and limbic leucotomy), vagus nerve stimulation (VNS), cortical stimulation techniques (such as transcranial magnetic stimulation [TMS]), and deep brain stimulation (DBS).
ELECTROCONVULSIVE THERAPY
Electroconvulsive therapy (ECT) is discussed in detail elsewhere in this volume (see Chapter 45). ECT has been used to treat depression since the 1930s, and many consider it the “gold standard” of antidepressant treatment. ECT involves delivery of an electrical current to the brain through the scalp and skull in order to induce a generalized seizure. While the mechanism by which generalized seizures alleviate depressive symptoms is not fully understood, the efficacy of ECT for depression has been demonstrated in a large number of clinical trials. A recent meta-analysis that included most of these clinical trials found that active ECT was significantly more effective than sham ECT and more effective than pharmacotherapy.4 However, many patients relapse unless they receive periodic maintenance treatments and there are common side effects, such as memory loss, that are associated with ECT.5
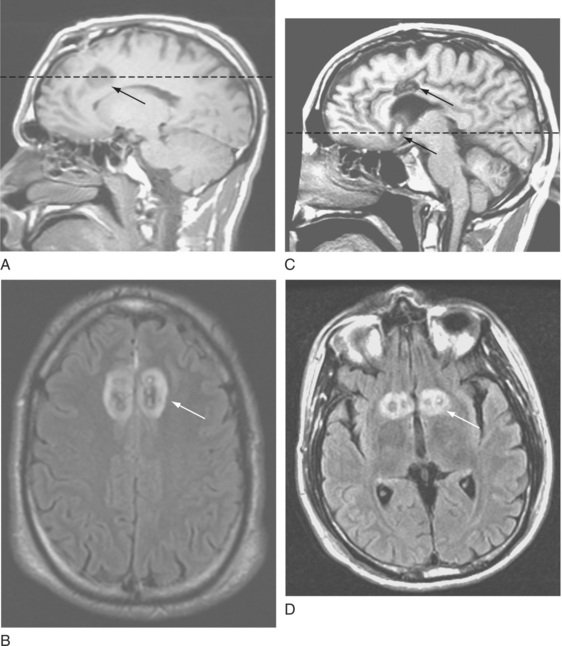
ABLATIVE LIMBIC SYSTEM SURGERY
Concerns regarding ablative neurosurgery for psychiatric indications are understandable given the indiscriminant use of crude procedures, such as frontal lobotomy, in the middle of the twentieth century. These procedures were associated with severe adverse events, including frontal lobe symptoms (e.g., apathy) or even death. In the latter half of the twentieth century, neurosurgeons began to use much smaller lesions in well-targeted and specific brain regions. As a result, the incidence of adverse events dropped precipitously. Currently used procedures include anterior cingulotomy, subcaudate tractotomy, limbic leucotomy (which is a combination of an anterior cingulotomy and a subcaudate tractotomy), and anterior capsulotomy (Figure 46-1). All of these procedures use craniotomy techniques. However, because of the small lesion volume required for an anterior capsulotomy, a gamma knife (a technique that uses focused gamma rays to create ablative lesions) can be used to perform this procedure. These procedures have been used in patients who suffer from intractable mood and anxiety disorders; modest response rates range from 30% to 70%.6–10 Because patients eligible for these procedures have failed all other available treatments, a significant positive response to these interventions can be lifesaving. While postoperative side effects may occur, they are almost always temporary. Inconvenient side effects include headache, nausea, and edema; more serious adverse events include infection, urinary difficulties, weight gain, seizures, cerebral hemorrhage or infarct, and cognitive deficits. Fortunately, these side effects are uncommon and typically transient.7,9,10
VAGUS NERVE STIMULATION
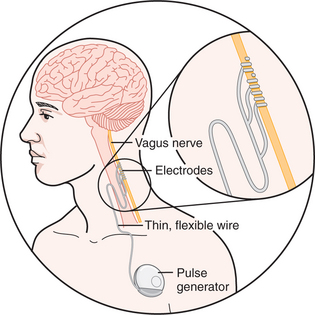
Vagus nerve stimulation (VNS) was approved for use in treatment-resistant epilepsy in 1994 in Europe and in 1997 in the United States. VNS has been approved for treatment-resistant depression (TRD) and bipolar disorder in Europe and Canada since 2001. In July 2005 the United States Food and Drug Administration (FDA) approved VNS for TRD. Implantation of the VNS device involves surgical placement of electrodes around the left vagus nerve via an incision in the neck (only the left vagus nerve is used for VNS because the right vagus nerve has parasympathetic branches to the heart) (Figure 46-2). A second incision is used to place an internal pulse generator (IPG) subcutaneously in the left subclavicular region, and the wire between the electrodes on the vagus nerve and the IPG is connected by means of subcutaneous tunneling between the two incision sites. After a 2-week postoperative recovery period, the IPG can be turned on and electrical stimulation of the left vagus nerve can be initiated.
Interest in studying the efficacy of VNS for TRD arose from the clinical experience of treating over 40,000 patients with treatment-resistant epilepsy with VNS. Depression is more prevalent in patients with epilepsy than it is in the general population, and it was noted that many patients with treatment-resistant epilepsy being treated with VNS experienced improved mood.11,12 Eighty percent of the fibers in the vagus nerve are afferent, meaning that an electrical charge delivered to the vagus nerve is predominantly sent to the brain. The left vagus nerve enters the brain and first innervates the nucleus tractus solitarius (NTS). While the mechanism of VNS is not completely understood, it is important to note that the NTS then communicates with the parabrachial nucleus (PBN), the cerebellum, the dorsal raphe, the periaqueductal gray (PAG), the locus coeruleus, and ascending projections to limbic, paralimbic, and cortical regions. Functional neuroimaging studies of subjects receiving VNS show increased cerebral blood flow in many of these brain regions that are implicated in the pathophysiology of MDD. For instance, the locus coeruleus and dorsal raphe nuclei contain the cell bodies of noradrenergic and serotonergic neurons that then project throughout the central nervous system. Last, the PBN communicates with other brain regions (including the hypothalamus, thalamus, amygdala, and nucleus of the stria terminalis) implicated in the pathophysiology of MDD.13
The three pivotal trials submitted to the FDA for approval of VNS for TRD were conducted to assess efficacy and safety. The first clinical trial was a randomized, controlled, acute 8-week study that assessed adjunctive VNS therapy versus sham treatment in patients with TRD.14 It is important to stress that VNS was studied as an adjunctive treatment. Therefore, it is recommended that all patients who receive VNS should continue to receive pharmacotherapy, psychotherapy, or both (and even ECT, if necessary). For the purposes of the clinical trials, ongoing treatment other than VNS was referred to as treatment-as-usual.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree