Fig. 1
Intraganglionic injection. Paravertebral surgical exposure for intraganglionic injection, illustrating the operative field (left panel), reference diagram (middle panel), and cleaned vertebral bones (right panel). (a) Initial dissection of soft tissues at the level of the fourth lumbar (L4) and L5 spinal nerves (yellow) shows the superior articular processes (sap), spinous processes (sp), and transverse processes (tp), as well as the laminar bone (lam) and accessory process on L4 (ap). The dorsal root ganglia are covered by laminar bone. (b) Removal of laminar bone superior to the foramen and the L4 accessory process reveals the distal dorsal root ganglion, recognized by its broader diameter and brownish-orange color. (c) The motorized injection system is mounted on a magnetic stand via a manual micromanipulator. The rat vertebral column is stabilized by clamping the spinous process of L5 using a clip mounted on an articulated arm that is attached to the same magnetic stand. The head of the rat is to the right. Reproduced with minor modification from Fischer et al. [5] with permission
4.
Make an underlying incision in the fascia of the paravertebral muscles just lateral to the midline.
5.
Use forceps to separate two layers of the paravertebral musculature by blunt dissection to expose the lateral aspect of the L6-L4 vertebrae, applying retraction using retractors mounted on magnetic holders as needed to maintain a clear view of the surgical field.
6.
Position the rat partially on to its side to achieve a better view of the lateral aspect of the L6-L4 vertebrae.
7.
Clean the lateral aspect of the vertebrae by blunt dissection to expose the L4/L5 and L5/L6 intervertebral foramina (IVFs), overlaid by a membrane of connective tissue, to reveal the L4 and L5 spinal nerves just distal (approximately 2 mm) from their DRGs, which are covered by laminar bone (Fig. 1a).
8.
Gently separate the membrane from the vertebral bone, exposing the entry of the spinal nerves into the IVFs. Pause as appropriate to control bleeding by compression with small balls of sterilized absorbent tissue paper.
9.
Use rongeurs to slightly enlarge the IVF and remove a 1 mm-deep crescent of laminar bone, exposing the distal third of the L4 and L5 DRGs (Fig. 1b) (see Note 4 ). Avoid compression of the DRG by using the smallest rongeur, taking small bites with only the tip of the rongeur, and maintaining a lifting force against the bone with the portion of the rongeur that is in contact with the DRG.
10.
Remove one aliquot of virus from −80 °C freezer. Thaw, flick the tube to mix, and then do a quick spin-down in a microcentrifuge (<2100 × g) to ensure no liquid is in the cap or on the walls of the tube.
11.
Load the pipette with injectate by drawing injectate back through the tip, taking care to avoid introducing air bubbles or cavitation, which can affect the accuracy of injection volume.
12.
Mount the rat in the clamp (Fig. 1c) and lift it such that the abdomen is just barely touching the table (to reduce venous bleeding). Physical support can then be provided under shoulder and hip with paper towel to stabilize the position.
13.
Approach the ganglion at as shallow an angle as possible (i.e., tangential to the surface of the capsule) to facilitate entry into the DRG without compression against the underlying bone.
14.
Slowly advance the pipette into the DRG to a depth of approximately 100–150 μm.
15.
Wait 2–3 min before beginning injection to allow the DRG capsule to close around the pipette tip.
16.
Gradually expel injectate (2 μl of AAV ) in small boluses, carefully watching for backflow from around the pipette tip (see Note 5 ).
17.
Injection of 2 μl of solution per DRG should take approximately 5 min.
18.
Once injection is complete, wait 5 min before withdrawing pipette, to allow pressure within the ganglion to equalize and minimize backflow.
3.4 Suture and Recovery
1.
After injections are completed, disengage the clamp and lower the rat to the table.
2.
After ensuring hemostasis, close the muscle fascia using absorbable suture.
3.
Close the skin with removable skin staples or wound clips.
4.
Once the incision is closed, discontinue the anesthetic, but continue to deliver oxygen.
5.
Keep the rat on the heating pad and monitor it continuously until the righting reflex is regained, at which point the animal can be transferred back to its cage.
3.5 Representative Results
1.
When following this protocol, a very precise injection with consistently successful transgene expression mediated by AAV is obtained. As a representative result of this method, we injected self-complimentary AAVs of various serotypes (5, 6, 8, and 9) into the L4 and L5 DRGs. Variable levels of EGFP expression were detected in the primary sensory neurons and their axons at 1 week following vector administration. A robust EGFP expression for all vectors tested was observed at 2–4 weeks after injection. EGFP expression was restricted to the sensory neuronal somata and projections, and no EGFP could be identified in satellite glia or other nonneuronal cells (Fig. 2).
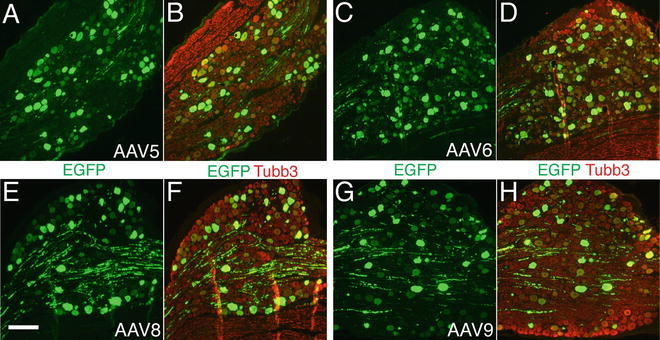

Fig. 2
Typical sensory neuron transduction after DRG injection of AAV5-, 6-, 8-, and 9-EGFP. Paraffin-sections from L5 DRGs were double-immunolabeled with the antibodies against GFP (green color) and neuron-specific β3-tubulin (Tubb3, red color). High-efficient transgene (EGFP) expression with predominant neuronal tropism is demonstrated in DRGs 4-week after intraganglionic injection of AAV5 (a and b), AAV6 (c and d), AAV8 (e and f), and AAV9 (g and h). Scale bar for all images: 200 μm
4 Notes
1.
All protocols using live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must be performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.
A highly purified AAV vector with a titer of at least 1 × 109 GC viral particles (injected) and >90 % purity by silver stain is recommended. Injecting less than 108 viral particles with low-purity per DRG may not lead to a desired in vivo transduction. AAV is generally considered nonpathogenic and regarded as a Biosafety Level 2 (BSL-2) material, and appropriate practices must be followed during AAV application.
3.




A 40–60 μm tip is suitable for dissolved compounds and suspensions of very small particles, such as viruses and nanoparticles. Larger openings may be necessary for suspensions of larger particles, i.e., cell suspensions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






