Fig. 1
Diffusion-based delivery results in a limited area of distribution and reflux along the needle track. Increased flow rate with CED results in a pressurized extracellular bulk flow that allows for a more homogeneous distribution of molecules/particles and increased distances from the cannula tip. Reflux is also reduced, especially with the step cannula design resulting in increased volume of distribution
CED was originally investigated predominantly for treatment of diseases such as glioblastoma because it offers several advantages, including of delivery of high concentration of molecules, robust distribution, targeted treatment, and lower system effects [5]. Since distribution is affected by hydrostatic pressure rather than diffusion, the molecular weight of the molecule is not usually the limiting factor controlling distribution. This supported the investigation of viral delivery into the CNS with the CED method.
CED was initially examined in gene therapy studies as a way to increase the distribution of rAAV2 vectors in the brain . Bankiewicz et al. revealed that CED can significantly increase gene transfer and distribution of rAAV expressing AADC in the striatum of MPTP-treated monkeys [8]. Similar results have been replicated by Cunningham et al. in the rat brain with rAAV2 expressing thymidine kinase (TK) [9]. The CED method showed robust gene transfer and increased distribution within the putamen, with immunoreactive cells found outside the striatum, including the globus pallidus, subthalamic nucleus, thalamus, and substantia nigra [9, 10]. Carty et al. examined the use of CED in mouse models [11]. They demonstrate that CED can be successfully used for different serotypes of rAAV for increased transduction of the mouse CNS .
Most recently a new method of real time convection delivery (RCD) has been established in order to make the method safer and more efficacious. This currently utilizes magnetic resonance imaging (MRI ) [12–14]. Viral samples can be co-administered with an MRI tracer such as gadoteridol or gadopentetic acid (Gd-DTPA) to monitor optimal placement of the injection. This also has the advantages of identifying if any reflux is occurring (especially at higher flow rates), or if the injected sample is leaking into the ventricles and to allow for corrections of flow rate or needle placement if necessary. In nonhuman primates, it has been shown that there is a direct correlation between gadoteridol distribution and AAV2 transduction [15].
Back flow failure using needles and catheters is a significant concern with injections into the CNS and this is one of the theoretical mechanistic limitations of the CED method as well. To limit this problem, Krauze et al. developed a step cannula design [6]. This can effectively reduce reflux by placing a silica tubing within the needle creating a horizontal step that reduces the back flow of fluid. The optimization of more efficient cannula designs coupled with the encouraging results from studies showing enhanced gene transfer and distribution emphasize the therapeutic potential of the CED method in helping overcome some of the mechanical disadvantages of gene delivery for gene therapy [6]. However, further refinements are required before CED becomes a standard clinical practice. CED is, however, an excellent method to achieve significant transduction of virus in research animals. This chapter continues to describe in detail the method used for CED delivery into a rodent brain .
2 Materials
2.1 Step Design Cannula
1.
Fused silica tubing (Polymicro Technologies, Pheonix, AZ, USA).
2.
Hamilton syringe with 27-gauge stainless steel blunt removable needle (RN) (Hamilton, Reno, NV, USA).
3.
Gel super glue.
2.2 Surgery
1.
Adult mouse.
2.
Preemptive analgesic.
3.
Isoflurane (Abbott Animal Health, Abbott Park, IL, USA).
4.
Sterile/germicidal surgical swabs.
5.
Iodine solution.
6.
Stereotaxic apparatus.
7.
Sterile adhesive plastic drape material (e.g., Bioclusive by Johnson & Johnson, or equivalent).
8.
#10 or #11 scalpel.
9.
Mosquito hemostats.
10.
Dremel drill with dental drill bit.
3 Methods
3.1 Step Cannula Assembly
1.
The step design cannula can be prepared as follows: fused silica tubing is partially inserted into a Hamilton 27-gauge stainless steel blunt removable needle (RN).
2.
A small amount of super glue is placed onto the silica tubing and the tubing is pushed further into the needle. Excess glue is dabbed off the needle. The glue is usually allowed 24 h to cure. In our experience, the gel type super glue seems to work more easily than the liquid type.
3.
After the glue has set, the end of the silica tubing is cut with a straight razor blade leaving 1–2 mm of tubing protruding from the end of the needle (Fig. 2; see Note 1 ).
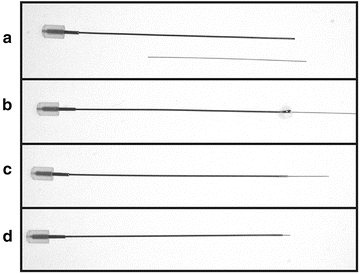

Fig. 2
Production of cannula designed needles. (a) Hamilton 27 gauge needle plus silica tubing are depicted. (b) Silica tubing is inserted in needle and gel super glue is applied. (c) Tubing is inserted slightly further to apply glue to inside of needle and excess glue is removed. (d) Silica tubing is cut to size, 1–2 mm extending from needle
3.2 Surgery
Our standard protocol is described, but minor variations in the surgical procedure to match local Institutional Animal Care and Use Committee requirements are easily incorporated. Figure 3 illustrates a typical stereotaxic set up for a mouse surgery (for rats the nose piece can be easily exchanged).
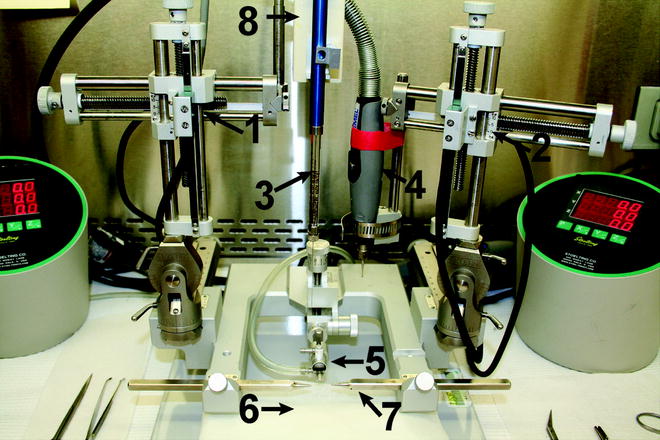

Fig. 3
A typical surgical stereotaxic setup. (1) Stereotaxic left arm, (2) Stereotaxic right arm, (3) Syringe with CED needle, (4) Dremel drill, (5) mouse nose piece for positioning mouse head and delivery of O2 and anesthesia, (6) warming pad to maintain mouse body temperature, (7) ear bars for positioning mouse head, (8) syringe pump for automated delivery. The oval control units on each side of the stereotaxic frame provide automated movement of the arms to the desired, preset coordinates, and are part of a digital system attached to the stereotaxic arms for accurate needle placement
In our experience maximal expression of transgene is achieved within 3–4 weeks post-injection. Transgene expression has been reported to continue for at least 1.5 years in rodents (most likely the life span of the rodent) and as much as 6 years in primates [16, 17]. The spread with serotypes such as rAAV9 into the hippocampus can target the majority of the entire hippocampus (Fig. 5). It should be noted that the level of expression and spread is dependent on the titer of the virus and in our experience also the transgene being expressed.
1.
The mouse is weighed, injected with preemptive analgesic, and placed in the anesthesia induction chamber connected to a calibrated vaporizer delivering isoflurane anesthetic.
2.
Anesthesia induction is usually performed with an initial vaporizer flow rate of 3–5 % with oxygenation at 0.5 L/min.
3.
Once anesthesia is evident, the mouse is removed from the induction chamber. The hair on the top of the head is shaved or plucked from the eyes rostrally to the nape of the neck caudally and to the ears laterally to prevent contaminating the operative site.
4.
The skin is cleaned three times by scrubbing with germicidal surgical scrub, wiping from the center of the surgical field outward. The surgical site is painted with a dilute, tamed iodine solution.
5.
The mouse is then moved to the surgery area, placed on an isothermal pad, and mounted in the stereotaxic apparatus.
6.




Anesthesia is conducted by surgical tubing into the surgical arena and delivered to the mouse using a rodent-specific nose cone apparatus (Fig. 3, arrow 5) and an anesthesia flow rate of 2 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






