Chapter 32 Acute Nerve Injuries
• Efficient identification of the injured nerve(s) and characterization of the nature and severity of injury facilitate the optimal evaluation and treatment of acute peripheral nerve injuries: obtaining a pertinent history and performing a directed physical examination are paramount. Each peripheral nerve is composed of fibers from more than one spinal nerve root; correspondingly, each spinal nerve contributes fibers to more than one peripheral nerve. Consequently, whereas spinal nerve lesions manifest in the clinical picture of radiculopathies with dermatomal sensory disturbances and mild to moderate weakness of muscles supplied by the spinal nerve, peripheral nerve lesions manifest in more precise borders of sensory disturbances acutely and more severe atrophy and paresis of muscles supplied solely by the peripheral nerve as time elapses.
• Use of electrodiagnostic and radiographic studies can augment the clinical evaluation when performed at the appropriate time and for specific indications. For example, the presence of intraoperative nerve action potentials (NAPs) across an involved segment implies that neurolysis alone is adequate for neural recovery.
• A preganglionic injury implies injury proximal to the dorsal root ganglion with permanent paralysis of the muscles innervated by the avulsed roots, complete sensory loss of the corresponding dermatomes, and most importantly, the preclusion of spontaneous recovery. A postganglionic injury potentially retains function of the cell body within the ventral horn of the spinal cord, and these neurons may regenerate axons in the appropriate conditions.
• Surgical options for nerve repair/reconstruction include neurolysis, nerve repair with and without graft, and nerve transfers. Direct (end-to-end) repair is possible only if a short nerve gap exists after resection of the nonfunctioning neural segment. Direct repair is preferred over indirect (graft) repair because of better functional results. Tension across the repair must be avoided to reduce the risk of failure of functional regeneration.
• Management of patient expectations is critical because recovery after nerve injury can take months to years. Nerve repair/reconstruction can be augmented with muscle/tendon transfers to maximize functional outcome.
Acute peripheral nerve injury may result from penetrating trauma, blunt trauma, compression, electrical and iatrogenic causes. The peripheral nerve is severed sharply in 30% of soft tissue lacerations,1 or it can remain grossly in continuity but with varying degrees of intraneural trauma from contusion and stretch. In 15% of nerve injuries associated with a potentially transecting mechanism, the peripheral nerve is only partially severed.2 Despite appearances, the injured peripheral nerve ultimately manifests in sensory and motor disturbances. The subsequent evaluation and treatment of acute peripheral nerve injuries rely upon the efficient identification of the nerve and the site affected and the characterization of the nature and severity of the injury. The challenge to the nerve surgeon remains in the optimal management of nerve injuries that will maximize functional outcomes.
Evaluating the Patient With an Acute Peripheral Nerve Injury
Anatomy and Physiology
A few basic principles underlie the anatomy of the peripheral nervous system. Each peripheral nerve is composed of fibers from more than one spinal nerve root; correspondingly, each spinal nerve contributes fibers to more than one peripheral nerve. Consequently, whereas spinal nerve lesions manifest in the clinical picture of radiculopathies with dermatomal sensory disturbances and mild to moderate weakness of muscles supplied by the spinal nerve, peripheral nerve lesions manifest in more precise borders of sensory disturbances acutely and more severe atrophy and paresis of muscles supplied solely by the peripheral nerve as time elapses.
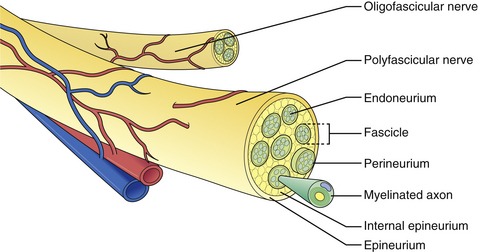
The microscopic anatomy of the peripheral nerve comprises nerve fibers, fascicles, connective tissue (epi-, peri-, and endoneurium), blood vessels, lymphatics, and nervi nervorum (Fig. 32.1). Although the fascicular pattern may change as the nerve courses more distally, the general structure of the nerve remains constant. After a peripheral nerve is injured, a coordinated sequence of events occurs to remove the damaged tissue and ultimately initiates the regenerative process. When the nerve is disrupted, the severed ends retract owing to the elasticity of the endoneurium. Trauma to the vasa nervorum leads to robust inflammation triggering fibroblasts to proliferate to form the basis for a dense scar at the injury site: in the most severe cases, the nerve ends become markedly disorganized, with fibroblasts, macrophages, capillaries, Schwann cells, and collagen fibers within which the regenerating axons form disorganized masses known as neuromas. The degree of damage sustained by the proximal segment and neuronal cell body depends on the distance of the zone of injury from the cell body. The nucleus migrates to the periphery of the cell and select cytoplasmic elements (e.g., Nissl granules, endoplasmic reticulum) undergo chromatolysis. Cell survival relies upon the Schwann cells and trophic molecules present in the immediate environment.
When a peripheral nerve is injured, the accompanying muscle is denervated. Denervation leads to a series of structural changes and atrophy of the muscle if neural regeneration does not occur. Atrophy is seen as a mean 70% reduction in the cross-sectional area after 2 months.3 Sodium channels regress toward embryonic forms with altered biochemical properties, and acetylcholine receptors redistribute to cover the entire muscle surface. This supersensitivity to acetylcholine manifests clinically as spontaneous uncoordinated muscle activity, otherwise known as fibrillation. Death of muscle fibers generally does not occur, but when it does, dropout occurs between 6 and 12 months after denervation.
Pertinent History of Injury
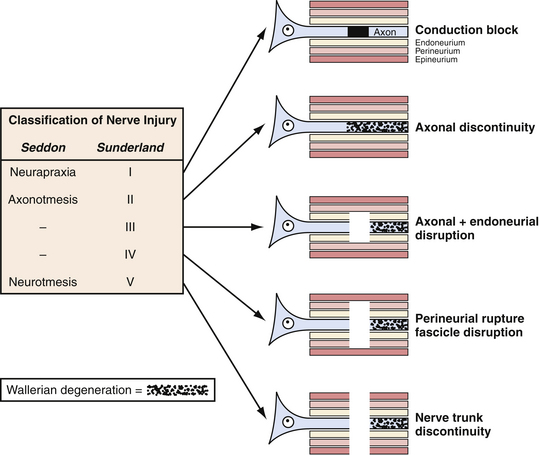
The description of the mechanism of injury can also yield information about the severity of injury to the nerve and guide treatment. For instance, if the injury involved low/no impact such as a fall from a standing position, neurapraxia will be the likely result. In contrast, if there is high impact as in extreme sports or high-speed motor vehicle accidents, then the likely injury is axonotmesis or neuronotmesis. This classification of nerve injury described by Seddon and associates in 19434 and expanded by Sunderland and colleagues in 19515 (Fig. 32.2) remains useful today, although most injuries occur along the continuum of pure grades.
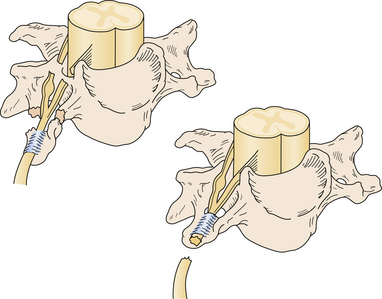
Nerve injuries can also be classified as preganglionic or postganglionic, with profound surgical implications (Fig. 32.3). A preganglionic injury implies injury proximal to the dorsal root ganglion with permanent paralysis of the muscles innervated by the avulsed roots, complete sensory loss of the corresponding dermatomes, and most importantly, the preclusion of spontaneous recovery. A postganglionic injury potentially retains function of the cell body within the ventral horn of the spinal cord and these neurons may regenerate axons in the appropriate conditions. Acute nerve injuries can be classified as open or closed. Open injuries include sharp lacerations and missile wounds whereas closed injuries include traction or compressive injuries. Current guidelines recommend early repair (days) of clean, sharp nerve lacerations, and the delayed (weeks) repair of ragged or dirty nerve lacerations. Missile wounds with vascular injury should be explored acutely. Early intervention is generally not recommended in closed injuries because of the potential for neurapraxic injury. Spontaneous regeneration, when it occurs, generally yields superior functional outcomes when compared to iatrogenic repair or reconstruction. Consideration of the clinical implications of these issues led to an algorithm for the surgical management of peripheral nerve and brachial plexus injuries6 (Fig. 32.4).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree